Researchers Develop Novel, Sustainable PFAS Defluorination Technique
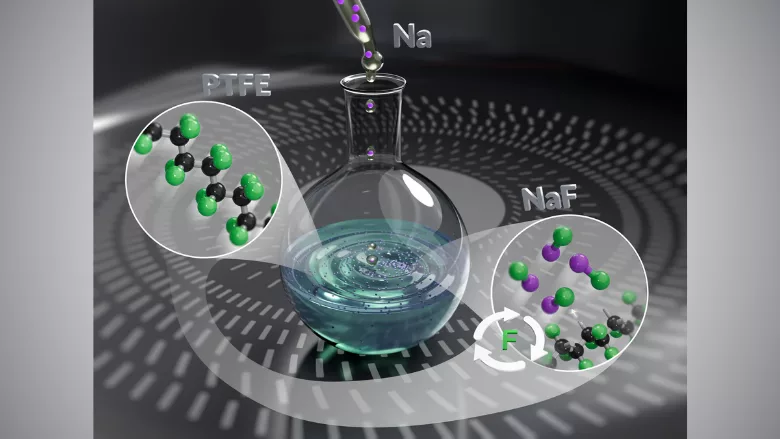
An international team of researchers has developed a novel method for defluorination polytetrafluoroethylene (PTFE) and other per- and polyfluoroalkyl substances (PFAS), which overcomes limitations of traditional high-energy or high-temperature defluorination processes.
The technique enables room-temperature defluorination with high fluorine recovery, using sodium dispersion as a key reagent.
PTFE is widely used in applications such as non-stick cookware and grease-proof food packaging. However, the “forever chemical” poses environmental and human health risks, as it does not break down and accumulates in soil, water, crops, animals, and people.
Traditional defluorination methods—in which PTFE is converted into its constituent fluorine compounds, allowing for polymer recycling—typically require high temperatures or complex reagents, and do not prioritize fluorine recovery.
To address these limitations, a team of researchers led by Norio Shibata, Ph.D., Professor of Chemistry at the Nagoya Institute of Technology (NITech) in Japan, developed a sodium dispersion-based approach that achieves defluorination at 25 °C in just 12 hours. The major advantage of the novel defluorination approach is that it can operate at room temperature, avoiding the need for extreme conditions.
Additionally, under optimal conditions, the method yielded up to 98 percent fluoride ion recovery in the form of sodium fluoride. Spectral analysis of the resulting black residue confirmed that approximately 93.5 percent of fluoride was successfully extracted from PTFE.
Further characterization using X-ray diffraction, Raman and infrared spectroscopy, and nuclear magnetic resonance validated the efficiency of fluoride recovery. Morphological analysis revealed a transformation in PTFE’s structure, from a dense, grainy texture to a rough, cracked surface, indicating successful degradation.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
The method also proved effective for other PFAS compounds of concern, including perfluorononanoic acid (PFNA), perfluorooctanoic acid (PFOA), perfluorobutanesulfonic acid (PFBS), and trifluoroacetic acid (TFA). With proper adjustments to reaction time and reagent quantity, fluorine recovery rates reached up to 97 percent.
This advancement not only supports the recycling of fluorinated polymers but also contributes to reducing reliance on fluorite, a finite resource. By avoiding high-energy processes and minimizing toxic emissions, the new defluorination technique represents a significant step toward the sustainable management of PFAS materials.
The research was published in Nature Communications.









.webp?t=1721343192)