Study Shows Irradiation with Zinc Oxide Nanocrystals Effectively, Sustainably Breaks Down PFAS

In a new study published in Chemical Science, researchers have demonstrated the promise of a new zinc oxiode (ZnO) nanocrystal-based defluorination technique for perfluorooctanesulfonic acid (PFOS), a significant type of per- and polyfluoroalkyl substance (PFAS), potentially solving recycling challenges presented by PFAS.
PFAS, often referred to as “forever chemicals” due to their persistence in the environment and human body, are used in industrial and consumer goods applications, such as nonstick cookware and food packaging, for their water-, grease-, and heat-resistant properties. However, these chemicals have come under increasing scrutiny as evidence about their harms to human health come to light—such as increased risk of cancers, liver and heart damage, and immune and developmental damage in infants and children—while they continue to accumulate in water, soil, food, animals, and people.
PFAS are made up of a chain of carbon and fluorine atoms, which gives the chemicals their stability and contribute to their inability to degrade naturally, due to the extremely high energy requirement of breaking the carbon–fluorine (C–F) bond.
Defluorination, which is the process of removing fluorine atoms from molecules, can make the chemical less stable and more susceptible to further breakdown, but traditional PFAS defluorination techniques are challenging as they require harsh chemicals or high energy. Therefore, novel, sustainable, and energy-efficient methods are required to enable PFAS to be recycled and mitigate PFAS-associated environmental risks.
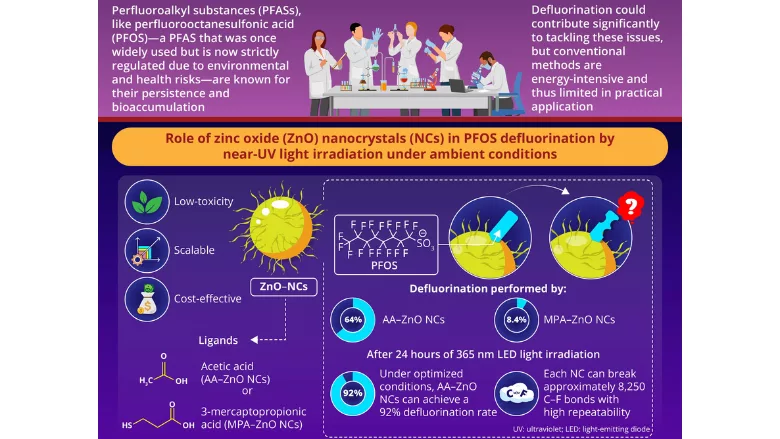
Addressing this gap, a research project led by Professor Yoichi Kobayashi, Ph.D. and Shuhei Kanao from Ritsumeikan University, Japan, explored the use of ZnO nanocrystals (NCs) in the PFAS defluorination process. NCs, known for their photocatalytic properties, can use light to generate reactive species that degrade organic pollutants. The researchers used NCs capped with different ligands for enhanced efficiency.
The researchers chose PFOS as the target PFAS type as it was once widely used and is therefore present in the environment; however, PFOS is now tightly regulated in many countries.
The study mainly focused on the defluorination efficiency of ZnO NCs, capped with acetic acid (AA–ZnO NCs) or 3-mercaptopropionic acid (MPA–ZnO NCs) ligands. Other organic ligands were also used to cap the NCs for comparative analysis. The defluorination experiment was conducted using 365-nanometer (nm) LED light, as it mimics ambient lighting conditions. The defluorination effect of these ligand-capped NCs was also tested on select other PFAS like trifluoroacetic acid (TFA) and Nafion.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
AA–ZnO NCs could efficiently defluorinate PFOS by irradiation with near-UV light under ambient conditions. The presence of acetic acid ligand proved to be far more efficient than 3-mercaptopropionic acid, as MPA–ZnO NCs achieved only 8.4 percent defluorination after 24 hours, while AA–ZnO NCs exhibited up to a 92 percent defluorination rate after 24 hours under optimized conditions.
To ensure the sustainability of these NCs, their durability and decrease in catalytic efficiency over time were also tested. The findings suggested that the decomposition reaction proceeded over multiple cycles, with a single ZnO NC able to break up to 8,250 C–F bonds, pointing toward its reusability.
ZnO NCs can be efficiently used in the defluorination process due to their unique properties. They are low-toxicity, inexpensive, and can be produced at scale, unlike many previous catalysts. The reaction occurs at room temperature and does not require high-energy light sources, which can be costly, fragile, or hazardous. This mild photodegradation system is a potential solution to PFAS recycling and for remediation of PFAS pollution.









