Laboratory Management
How Simple Microbiological Truths Provide Insights for Understanding and Solving Practical Problems
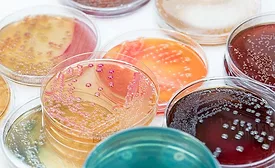
There is much in the tried-and-true past that informs us of our ability to progress with food safety and food quality microbiology
October 21, 2025
BIZTRACKS
SGS Expands With New Food Safety Testing Facility in New Jersey
October 18, 2024
Rapid Testing Methods—The Future
Most companies no longer have a microbiology lab or pathogen analysis capabilities, which will change the types of rapid test methods that will be in demand in the future
April 12, 2024
Never miss the latest news and trends driving the food safety industry
Newsletters | Website | eMagazine
JOIN TODAY!Copyright ©2026. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing