Something Old, Something New: Innovation within Existing Food Testing Technologies
In an earlier article, A Summary Profile of Pathogen Detection Technologies (Food Safety Magazine, April/May 2009), three foodborne pathogen detection technologies were examined—conventional culture (the “gold standard”) and two prevailing rapid test methodologies (immunoassay and molecular biology). Comparisons were provided in terms of critical performance attributes—speed, accuracy and ease-of-use. Procedures and end-user costs for each technology were addressed. The article’s objective was to profile the value of pathogen testing within the broader context of food safety and process control programs, and provide the reader with a performance-based analysis of several available pathogen detection options.
Amid recurring food-related public health issues, mounting consumer expectation and regulatory food safety requirements, coupled with a relentless industry drive for competitive advantage, an environment has emerged in which food safety failure is not a commercial option. Linking a product with a foodborne pathogen, resulting in consumer illness, is a catastrophic event for the food processor; few recover from a single incident.
On a brighter note, this evolving environment has also promoted the development and commercialization of new and improved pathogen detection capabilities. This follow-up article will focus on two recent technology developments in the rapid immunoassay and molecular methods areas—the magneto immuno-chromatography test (MICT) and loop-mediated isothermal amplification (LAMP). Both appear to deliver improvements in the key areas of detection speed and accuracy, ease-of-use and cost-effectiveness. While each maintains “one foot in conventional detection technology” (immunology or molecular biology), their respective innovations may provide superior solutions to ongoing food safety and process control challenges. The article closes with a suggested framework for test method assessment and selection.
Innovations in Immunology and Molecular Biology
MICT (Figure 1) consists of antibody-coated superparamagnetic nanoparticles in a lateral flow immunoassay format. The system includes a highly sensitive, low-cost magnetic reader for automated, non-subjective detection of the target. If the result is positive, then system software can also back-calculate the original contamination level, based on sample enrichment T-values (time), producing both qualitative (presence/absence) and quantitative (original cell count) results. The MICT innovation reflects a well-established ELISA technology for pathogen detection, achieving capture of the target through antigen/antibody binding affinity, but utilizes magnetic nanoparticles in the detection phase versus an optical conjugate/substrate enzyme reaction to produce color change or fluorescence.
Procedurally, the food or environmental sample is briefly enriched, then heat-killed for user safety. An aliquot of the heat-killed enrichment is placed in the MICT device. As the sample flows through the device, antibody-coated supermagnetic nanoparticles bind the target antigen if present. Bound nano-particles are subsequently captured by additional affixed antibodies in a downstream stage of the device, creating a classic immunoassay “sandwich.” When the device is placed into the reader or magnetometer, the presence of bound nanoparticles is detected, resulting in a positive result. System software enables test results to be downloaded into an Excel format to the user’s laptop or laboratory information management system for further data management.
An AOAC “Performance-Tested Method” for E. coli O157:H7 in the MICT format (Method #060902) is currently available in North America. In terms of its critical performance attributes, the following data have been published by the AOAC Research Institute. Internal and independent studies of E. coli O157:H7 detection via MICT at 1 CFU/g in 25-g and 375-g ground beef samples compare well with both cultural and molecular reference methods.
The MICT format requires a 6-hour sample enrichment at 39 °C in commercially available media. Finished results are available within an hour of sample enrichment and at sensitivity and specificity levels greater than or equal to 98%. The procedure appears to require technical expertise no greater than standard Good Laboratory Practices (GLPs). It is believed that the method’s limit of detection (LOD) is 100 to 1,000 times better than current immunoassay or molecular offerings. This lower LOD would account for the reduced sample enrichment time required, enabling the generation of an accurate test result within a single production or lab shift. The improved time-to-result may benefit both operations and quality assurance (QA) by accelerating raw and finished product release, reducing product hold times and providing an earlier indication of emerging process control issues.
Published pricing data indicate instrument and consumables costs are in the neighborhood of $10,000 and $10, respectively. This represents a sharp decrease in system instrument cost versus many automated test systems, while volume-dependent cost-per-test appears consistent with other rapid methods. Superior speed, comparable accuracy, ease-of-use and reduced overall cost would support further consideration of the diagnostic innovation afforded by MICT technology and its potential impact on food processing. MICT-based rapid assays for additional foodborne pathogens are currently under development, with anticipated completion dates in 2010.
LAMP represents a process innovation in DNA-based testing methodologies, specifically in the area of polymerase chain reaction (PCR), the technology basis for most current molecular methods. To achieve exponential DNA amplification and enable rapid, accurate detection of the target analyte, current PCR-based methods utilize thermocycling. As the temperature of the enriched sample aliquot is increased, DNA strands within it denature. As the temperature is reduced, specific primers bind the single target DNA strands and amplification occurs. This process is repeated hundreds of times to amplify the target DNA to a detectable level.
The temperature cycling and DNA amplification process, enabled by PCR, is conducted within a thermocycler. The process innovation that LAMP technology provides is the amplification of target DNA at a static temperature (63 °C). This is achieved by the utilization of multiple primers, including loop primers and Bst polymerase, resulting in an amplification rate of 109 target nucleotides (i.e., 1 billion DNA copies) within 15–60 minutes at a single, static temperature. Because the process is isothermal, a simple, inexpensive heating block, accommodating 32 sample tubes simultaneously, replaces the thermocycler.
A by-product of LAMP amplification is magnesium pyrophosphate, resulting in increased sample turbidity. Optical sensors within the turbidimeter (Figure 2) monitor sample turbidity at 6-second intervals. As the turbidity threshold is exceeded, indicating amplification of the target DNA, the LAMP-based system reports a positive result, often in as little as 15 minutes. If there is no turbidity increase within an hour, the system reports a negative result. The system software resides in a dedicated laptop, providing additional data management capabilities.

As with all test methods, sample enrichment is required prior to running the 1-hour amplification/detection procedure. Independent LAMP technology performance studies conducted in 2009 report an LOD of 104 CFU in the enriched sample, comparable to other molecular methods, and statistically equivalent or better sensitivity and specificity levels. One LAMP-based system manufacturer has an enrichment optimization project under way to reduce sample enrichment times to less than 8 hours. LAMP-based assays for E. coli O157:H7, Salmonella, L. monocytogenes and Campylobacter are currently available. Several tests have been awarded Japanese Ministry of Health approvals. Others are also currently involved in the AOAC approval process.
In terms of critical performance attributes, much can be said for LAMP’s molecular method innovation. The amplification/detection process has been reduced to less than 1 hour. Whatever the manufacturer’s enrichment optimization project achieves will ultimately determine LAMP’s bottom-line speed equivalence or advantage. Independent studies and Japanese Ministry of Health approvals support equivalent accuracy to cultural and molecular reference methods, while the pending AOAC approval process will further reinforce those findings. Multiple independent research papers presented at the International Association for Food Protection’s 2009 Annual Meeting support the technology’s viability.
The LAMP test procedure indicates no technician requirement beyond GLPs, consistent with all molecular methods. Replacement of the PCR thermocycler with the LAMP turbidimeter suggests a significant reduction in instrument and start-up costs (e.g., $15,000 vs. $50,000). It is believed that the cost-per-test for reagents in the volume-dependent $8–12 range is comparable to other molecular methods.
Assessing and Selecting Test Methods
With multiple current and emerging pathogen test method options available, what is the best approach to making a qualified assessment and selection? Two globally recognized food safety authorities, William H. Sperber, Ph.D. (Cargill) and J. Stan Bailey, Ph.D. (bioMérieux), have provided clear and consistent guidance on this topic. Both have suggested six critical method selection criteria: speed (how quickly can you access reliable data and take appropriate action—release or reject, rework or re-clean, etc.), accuracy (sensitivity, specificity, repeatability, etc.), ease-of-use (level of technical expertise required to operate), total cost (system instruments and lab equipment required, consumables and disposables, labor, etc.), method approvals (AOAC, ISO, etc.—how well does the candidate method compare to the reference method?) and, finally, sample number and type (volume throughput capacity on food matrices relevant to your operations). Careful consideration of these six criteria will assist in the selection of the most appropriate method for the user’s unique needs and capabilities.
Once one or several methods have been identified, consistent with the selection criteria, two additional qualifying steps should be taken prior to final test method selection—vendor and system assessment. Contact each test method supplier and examine their organizational capabilities. What level of sales and technical support is available? Will initial on-site technician training be provided and how will future system users be trained? In the event of system failure, what remedial steps will be taken and how quickly (e.g., immediate system replacement or repair)? How much lead time is required for consumables orders (e.g., test kits, sample enrichment media, etc.)? Also, processors should solicit customer references for independent performance and reliability input. How do others rate the vendor’s customer responsiveness?
After qualifying vendor capability, customer commitment and reliability, the final critical step is to analyze how well the test methods fit into the processor’s workflow and perform on their unique products. This analysis should be conducted jointly between the processor’s operations and QA teams. Contact the test method supplier(s) and request an on-site system trial. Most vendors will provide system installation, on-site training and support, as well as a reasonable number of test kits at little or no cost to the evaluator.
The system evaluation should focus on two areas—workflow and sample matrices. How well does the test procedure accommodate production and laboratory schedules? Can the method be integrated well into current processes or will workflow changes be required? Most importantly, does the method perform well on each and all of the processor’s product and environmental sample matrices? If there are sample matrix limitations, what other accommodations will be required? The vendor and system assessment will provide invaluable information in making the final test method
selection.
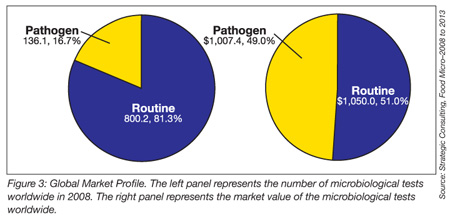
Amid ever-increasing consumer, regulatory and food processor urgency to address and resolve food safety issues, a corresponding increase in value-added foodborne pathogen detection options is also evident. New test methods continue to evolve from both existing and emerging diagnostic technologies, all focused on a global market of 138 million annual foodborne pathogen tests, with a total market value in excess of $1 billion and expanding at an annual rate of 8%, as identified in Strategic Consulting Inc.’s Food Micro 2008 to 2013 report. This article has examined two new methodologies, MICT and LAMP, each representing innovations to existing technologies that deliver improved speed, accuracy, ease-of-use and cost-effectiveness—something old, something new. Selecting the right test method for a food processor’s unique and specific applications can be achieved through an orderly, cross-functional approach, focusing on several critical performance criteria and integrating all stakeholders in the process, including QA, operations and the method’s vendor.
A. Crispin Philpott is a food safety consultant in West Chester, PA. He can be reached at 610-696-4405 and acphilpott@aol.com.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →








