The Foreign Supplier Verification Program: Boon or Bane?

As the population of the U.S. becomes ethnically more diverse and their appetite for a variety of foods becomes insatiable, there is a growing need to import a greater variety of food products. The U.S. imports food products from more than 90 countries. More than 20 percent of the total food supply in the U.S. is imported (70% of seafood and 35% of fresh produce available in the U.S. are from other countries). Of these importers, 70 percent are small business entities. The data in Tables 1 and 2[1] provide information about the value of imports from the top 10 importing countries and the value of food product categories coming through U.S. ports, respectively.
High-profile food safety incidents from 2000 to 2010 prompted Congress to implement a series of regulations in 2011—the Food Safety Modernization Act (FSMA)—to protect the food supply chain in the country. Details of the seven rules of FSMA were published widely. According to U.S. Centers for Disease Control and Prevention statistics, foodborne illness strikes 48 million Americans each year, requiring hospitalization for more than 120,000 people and resulting in approximately 3,000 fatalities, particularly for vulnerable groups. The intent of the seven rules of FSMA is to comprehensively and proactively prevent food safety incidents in the U.S. supply chain.
FSMA represents a sea change for food safety, not only in the U.S. but also globally. The focus is on prevention. It will have a dramatic and progressive effect on the safety of the food supply in the U.S. The most important regulation for food importers is the Foreign Supplier Verification Program (FSVP), published on November 27, 2015, with an initial compliance date of May 30, 2017. It contains a new set of requirements for importers.
This article is about the impact of the FSVP on the food production and processing entities in the domestic and foreign arenas, and the challenges that importers face.
What Are the Key Requirements of the FSVP?
The details of the FSVP have been widely published in the print media. However, in the opinion of the author, there is a big gap in understanding, particularly in the food importer community. Therefore, a review of the requirements will be appropriate here. According to the rule, importers are responsible for ensuring the safety of food products they bring into the U.S. for distribution and sale for consumption by the public. The FSVP rule requires importers to perform risk-based activities to verify that food imported into the United States is not adulterated [Section 402 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) of 1938] or misbranded with respect to allergen labeling (Section 403 of the FD&C Act) and has been produced in a manner that meets applicable U.S. product safety standards. The rule is flexible in the sense that importers have the flexibility to determine the appropriate verification measures for the foods they import based on food and supplier risks.
The FSVP applies only to importers of food products in the U.S. By definition, an importer is the U.S. owner or consignee of a food offered for import into the United States. If there is no U.S. owner or consignee, the importer is the U.S. agency or representative of the foreign owner or consignee at the time of entry, as confirmed in a signed statement of consent.
The specific responsibilities of importers with respect to food safety include:
• Determining known or reasonably foreseeable hazards with each food
• Evaluating the risk posed by a food, based on the Hazard Analysis, and the foreign supplier’s performance
• Approving suppliers and determining appropriate supplier verification activities based on the risks posed by an imported food and the supplier’s performance
• Conducting supplier verification activities and taking corrective actions for deviations and discrepancies
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
It is a requirement that importers import foods only from approved foreign suppliers based on an evaluation of the risk posed by the imported food and the supplier’s performance. However, it does not mean that importers cannot import food products from unapproved suppliers on a temporary basis, as long as these products are subjected to adequate verification activities before importation. Per the FSVP, importers are required to develop, maintain, and follow an FSVP for each food brought into the U.S. from each foreign supplier of that food. This implies that if an importer imports a certain food from a few different suppliers, a separate FSVP would be required for each supplier. Similarly, if an importer imports many different foods from a single supplier, a separate FSVP would be required for each food.
It is also likely that certain importers are also manufacturers/processors. The question is: What is the applicability of the FSVP in such cases? These entities will be in compliance if:
• the importer/manufacturer complies with the supply chain program requirements of the preventive controls rules; or
• the importer/manufacturer implements preventive controls for the hazards in the food as per the requirements of preventive controls rules; or
• the importer/manufacturer is not required to implement preventive controls under specified circumstances, such as when the type of food could not be consumed without application of a preventive control or when the customer of the importer-manufacturer will minimize or prevent the identified hazards.
Importers must evaluate the risk posed by the imported food and the supplier’s performance every 3 years and keep records of reviews. Evaluation of the identified risks is also mandated when new information emerges about a potential hazard or the foreign supplier’s performance. If the importer receives adequate assurances that a subsequent entity in the distribution chain, such as the importer’s customer, is processing the food for food safety in accordance with applicable FSMA rule requirements, then reevaluation of product risks and supplier performance is not required.
To proactively mitigate or eliminate the hazards in imported foods, an importer is required to identify and evaluate the known or reasonably foreseeable hazards for each type of food to determine whether there are any hazards requiring control and document them. The importer is also required to assess the vulnerability of materials/products to food fraud. Importers will have to produce such documentation when required during an inspection by the U.S. Food and Drug Administration (FDA).
Another important requirement of the FSVP rule is that importers must evaluate the performance of their foreign suppliers periodically. It should include foreign manufacturers’ Hazard Analyses, entities responsible for controlling hazards (foreign manufacturer or their suppliers), their food safety procedures, processes, and practices, their compliance with the applicable regulations, and food safety history. It is also possible for the importer to rely on another entity (other than the foreign supplier) to perform an evaluation of risk, so long as the importer reviews and assesses the relevant documentation.
It is again the responsibility of the importer to verify their foreign supplier on a regular basis. You may want to know what type of verification activities would comply with the FSVP requirements. The FSVP rule provides adequate flexibility for importers to meet the requirements that are unique to the products and supplier characteristics. Some of the accepted verification activities are annual on-site audits of the supplier’s facility, sampling and testing, review of the supplier’s relevant food safety records, etc. Annual on-site audits of the supplier’s facility are required when there is a reasonable probability that exposure to a hazard controlled by the foreign supplier will result in serious adverse health consequences or death to humans or animals (a SAHCODHA hazard). However, the importer can also choose other means of verification, provided the alternate choice will ensure that the foreign supplier is producing the food in accordance with applicable U.S. safety standards. An importer can also rely on another entity to determine and perform appropriate supplier verification activities, so long as the importer reviews and assesses the relevant documentation.
Taking appropriate corrective action by importers is the last piece of the puzzle in meeting the FSVP requirements. At times, an importer’s verification activities may provide evidence that a foreign supplier has not provided the same level of public health protection as required under the Produce Safety and Preventive Controls rules. This should promptly trigger corrective actions at the importer’s end. The appropriate corrective measure will depend on the circumstances and may include discontinuing the use of the foreign supplier.
The FSVP rule requires importers to provide the name, email address, and unique facility identifier (UFI) for each line entry of food product offered for importation into the United States. FDA has recognized the data universal numbering system (DUNS) number as an acceptable UFI for the FSVP. If the importer is temporarily unable to obtain the DUNS number, FDA intends to temporarily allow filers to transmit the value “UNK” (“unknown”) in the UFI field. This option began May 30, 2017, so that food offered for import could be processed through the Customs and Border Patrol automated commercial environment system, even if the importer has not yet provided a DUNS number.
Are There Exemptions from FSVP Rules?
Importers of the following categories of food products do not have to comply with the FSVP rule:
• Juice, fish, and fishery products subject to and in compliance with FDA’s Hazard Analysis and Critical Control Points (HACCP) regulations for those products, and certain ingredients for use in juice and fish and fishery products subject to the HACCP regulations
• Food for research or evaluation
• Food for personal consumption
• Alcoholic beverages and certain ingredients for use in alcoholic beverages
• Food that is imported for processing and future export
• Low-acid canned foods (LACF), such as canned vegetables, but only with respect to microbiological hazards covered by other regulations, as well as certain ingredients for use in LACF products (but only with respect to microbiological hazards)
• Certain meat, poultry, and egg products regulated by the U.S. Department of Agriculture (USDA) at the time of importation
Who Is Eligible for Modified FSVP Requirements?
In certain cases, importers need not comply with all the FSVP requirements. The criteria for modified FSVP requirements are listed below.
Dietary supplement importers: Importers complying with the requirements of 21 C.F.R. Part 111 (Current Good Manufacturing Practices) regulation will be required to comply with the modified requirements. Importers of other dietary supplements would be required to comply with the standard FSVP requirements (except the Hazard Analysis requirement).
Very small importers and importers of food from certain small suppliers: The definition of “very small importer” is annual sales of $1 million for human food and $2.5 million for animal food (averaged over a 3-year period) combined with the U.S. market value of food that is imported, manufactured, processed, packed, or held without sale (e.g., imported for a fee). In this case, importers would not have to conduct Hazard Analyses and would be able to verify their foreign suppliers by obtaining written assurances from their suppliers.
Importers of certain small foreign suppliers are subject to modified FSVP requirements: Those small suppliers are:
• Facilities subject to modified requirements under the Preventive Controls rules because they are qualified facilities
• Farms that are not covered farms under the Produce Safety rule because they average $25,000 or less in annual produce sales or because they meet requirements for a qualified exemption
• Shell egg producers with fewer than 3,000 laying hens
• Products from countries whose food safety system has been recognized as comparable or determined to be the equivalent of the U.S. system (e.g., Canada, New Zealand, Australia)
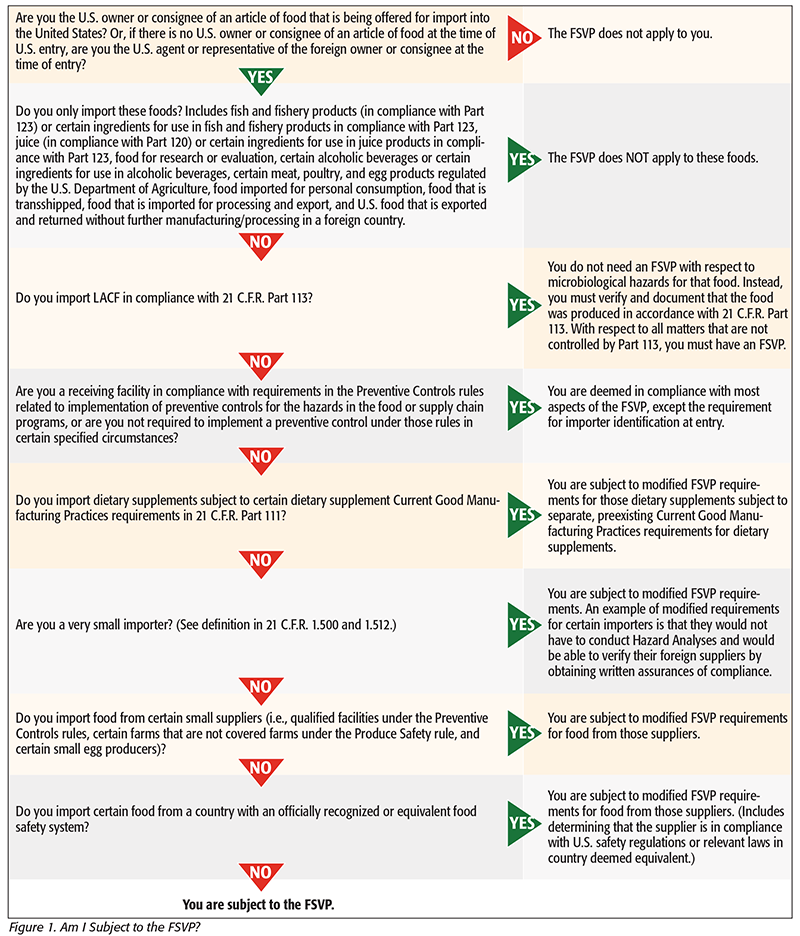
Please verify the applicability of the FSVP rule to you using the flowchart in Figure 1.
How to Develop and Implement a Robust FSVP?
Although FDA had been working on the FSVP rule for quite some time, the requirements caught many an importer off guard, specifically, those pertaining to developing food safety plans to control the safety of products they import. Many importers simply do not have the technical expertise or resources.
Traditional FDA inspections are production-centric. FSVP inspections, on the other hand, will be based on review of records, according to Sharon Mayl, senior adviser for policy in the Office of Foods and Veterinary Medicine at FDA. Further, while most of the FSVP inspections will be at the importer’s place of business, FDA may also request importers to provide FSVP records electronically, or by other means, to get them promptly. During the initial stages of FSVP implementation, FDA inspectors may review records and provide importers opportunities to correct them to support compliance. However, this approach will not be applicable to problems that pose a danger to health or reflect intentional disregard for legal responsibilities, according to Mayl.
A six-step approach to implementing an effective FSVP is shown below.
Step 1: Determine type of food/food categories to be included in the FSVP
The first step is to determine what food or food categories are to be included in the FSVP. FSVP requirements are risk based and depend on the type of food, type of hazard, and performance of suppliers. Per the FSVP rule, an importer is required to prepare an FSVP for each imported food or food category from each facility and each country. This is because the potential hazards requiring preventive controls may be specific to the type of food or food category from each facility and from each country. It may be possible to combine products and include them all under one FSVP, provided the hazards are the same or similar. However, you are still expected to prepare different FSVPs for the various facilities and various countries from which you import these products. If this is not clearly understood, you may get into issues with FDA.
Step 2: Assign responsibilities
As an importer, you must clearly understand the roles and responsibilities of the various entities, such as the importer, foreign supplier, and qualified individual (QI). Assigning responsibility does not absolve you from ensuring that the food you import is equivalent with respect to the safety of the products manufactured in the U.S. and will not pose any public health issues in the U.S. Your foreign suppliers may not be knowledgeable about the applicable Preventive Controls rules (human food or animal food), Produce Safety rule, or other relevant product-specific regulations. It is your responsibility to provide the relevant information to them.
You may request the foreign supplier or foreign manufacturer to assist you in preparing your FSVP by providing relevant information regarding the potential hazards and the control or mitigation strategies related to their products.
It is certainly a difficult task, at least for small importers, to meet such technical requirements of the FSVP rule without the help of a QI. If you can afford it, you may hire a full-time QI. Alternatively, you may seek an external resource to help you with the responsibilities. It is a requirement of the FSVP rule that certain activities must be carried out only by a QI.
Step 3: Implement QI responsibilities
A QI has the following responsibilities:
• Document and implement the Hazard Analysis
• Conduct performance evaluation of foreign suppliers
• Approve suppliers
• Document and implement verification activities
• Document and implement corrective actions
• Reevaluate FSVP and document reevaluation
• Maintain records
By definition, a food hazard is something that could cause illness or injury to humans or animals that eat the food. The first step in the FSVP is to evaluate known or reasonably foreseeable hazards for the food that is being imported to determine whether each of the potential hazards requires control.
Hazards can be biological, chemical, physical, unintentionally introduced, or intentionally introduced for economic gain (economic fraud).
The importer must use a QI to evaluate the hazards. It is quite acceptable for an importer to rely on someone else’s Hazard Analysis of the foreign supplier’s food so long as the importer’s qualified individual has reviewed the Hazard Analysis document and approved it. An importer’s Hazard Analysis is more than just accepting a Hazard Analysis of your foreign supplier.
It is your responsibility again to monitor the performance of your foreign suppliers. This process consists of examining their food safety procedures, processes, and practices. It should also include your verification of their compliance history with FDA food safety regulations and their food safety history. Further, your QI should document in the FSVP plan the entity that will be significantly minimizing or preventing the hazards identified by the Hazard Analysis, such as the foreign supplier or the supplier’s raw material or ingredient supplier, or perhaps a customer located in the U.S.
It is a requirement of the FSVP rule that importers approve their foreign suppliers before importing food from them. Again, you may use unapproved suppliers on a temporary basis, but only after ensuring the food is subjected to adequate verification activities before importation. You need to consider evaluation of the risk posed by the food, who is controlling the hazards, evaluation of foreign supplier performance, and other relevant factors in approving your foreign suppliers. Your QI will have to be responsible for this activity.
As an importer, you must monitor the food safety performance of your foreign suppliers. You have the flexibility to determine the type of verification activity for your foreign suppliers based on the risk posed by the products imported. FDA has identified the following supplier verification activities: on-site auditing, sampling and testing, review of supplier records, and other appropriate measures. Annual on-site audits of the supplier’s facility are required only when there is a reasonable probability that exposure to a food hazard controlled by the foreign supplier will result in a SAHCODHA hazard. However, the importer may choose other means of comparable verification to confirm the foreign supplier is producing the food in accordance with applicable U.S. safety standards.
As a result of the verification activities conducted by your QI, you may discover that your foreign supplier is not properly controlling the identified hazards. Should this happen, you are required to take action to correct the deficiency. The QI may be required to reevaluate the FSVP for the specific food and foreign supplier, depending on the deviations, and document them.
Reevaluation of your FSVP by your QI is required every 3 years or anytime you become aware of new information that may affect your prior evaluations.
FDA is going to rely heavily on records during inspections to determine your compliance with FSVP requirements. Therefore, ensure that your record maintenance procedures are robust. Failure to keep adequate records is a violation of the FSVP rule and the FD&C Act. FDA can take enforcement action in such cases. The list of records to be maintained includes the Hazard Analysis, the foreign supplier performance evaluation, procedures for approving foreign suppliers, foreign supplier approvals, procedures to ensure use of only approved foreign suppliers, determination and frequency of verification activities, performance of verification activities, corrective actions, and reevaluations of your FSVP. Records can be kept as original records, true copies, or electronic records.
Step 4: Determine applicability of other food safety requirements
Your foreign supplier may not be aware of the applicable U.S. food safety regulations. Your QI should determine whether other U.S. regulations apply to products that are imported. You should communicate such requirements to your foreign supplier so that the foreign supplier is in a position to comply with these requirements before the product reaches the U.S. port.
Step 5: Communicate effectively
The importance of communicating with your stakeholders cannot be overlooked. You must ensure effective communication at all levels in the supply chain, including your foreign suppliers, your clients/customers, and regulatory bodies. A good communication plan is all that is needed for effective communication.
Step 6: Be “FDA inspection ready”
This is the last step of the six-step approach to implementing a robust FSVP. It will automatically fall into place once you have taken care of the five previous steps.
Implementation of FSVP Rules and FDA Inspections
The Office of Regulatory Affairs of FDA releases inspectional observational summaries every year, providing information about the number of Form 483s issued to companies producing drugs, foods, veterinary medicines, biologics, medical devices, etc. A total of 5,045 Form 483s were issued from October 1, 2016, to September 30, 2017, out of which 2,662 were issued to food companies. One hundred eight Form 483s were issued against 21 C.F.R. 1.502(a) to importers for not developing an FSVP plan. It is already one of the top 20 violations in the food sector. Although the first compliance date for implementing the FSVP was May 30, 2017, it is interesting to note that FDA inspectors have already issued 108 Form 483s to importers out of a total of some 300 importers inspected in 6 months. It is quite likely that FDA in 2018 will ramp up the inspections of importers three to four times that of last year.
The second compliance date was March 19, 2018, for “small businesses” (foreign suppliers with < 500 full-time employees) and March 18, 2019, for “qualified facilities” and “very small businesses” (foreign suppliers with < $1 million in average annual sales). The compliance dates for importers whose foreign suppliers are subject only to the Produce Safety rule are July 29, 2019, for small business and July 27, 2020, for very small businesses. All other businesses must comply starting July 26, 2018. FSVP compliance dates are based on the size of the foreign supplier and not on the size of the U.S. importer.
Recent FDA FSVP Guidance Documents
Recently, FDA has released the following FSVP draft guidance documents:
Application of the FSVP Regulation to the Importation of Live Animals: Guidance for Industry, March 2018
This guidance document provides clarification regarding the applicability of the FSVP rule for the importation of live animals. The food resulting from the slaughter and processing of certain live animals cannot be consumed without slaughter and processing at establishments subject to USDA-administered HACCP requirements (or equivalent state programs). FDA has clarified that FSVP importers of live animals that are slaughtered and processed at USDA-inspected establishments subject to USDA-administered HACCP requirements (or state-inspected establishments subject to equivalent requirements) do not have to meet any of the FSVP requirements.
Application of the FSVP Regulation to Importers of Grain Raw Agricultural Commodities: Guidance for Industry, January 2018
Many raw agricultural commodities (RACs) that are not fruits or vegetables, including grains, are imported into the United States. The importation of grain RACs into the United States is subject to certain supplier verification requirements established in FSMA. FSMA amended the FD&C Act to add, among other food safety requirements, provisions requiring the verification of the safety of food imported from foreign suppliers of that food.
To better align the FSVP regulation with the exemption from preventive controls requirements for facilities solely engaged in the storage of nonproduce RACs, and because of the nature of the hazards associated with grain RACs and how they are generally addressed in the distribution chain, FDA intends to exercise enforcement discretion for importers of grain RACs that are solely engaged in the storage of grain intended for further distribution or processing [in accordance with 21 C.F.R. 117.5(j) or 507.5(g)] with respect to the FSVP regulation. This means that FDA will not expect the FSVP importers of grain RACs (i.e., grain elevators and other facilities solely engaged in the storage of grain RACs intended for further distribution or processing) to meet any of the FSVP requirements. However, these grain RAC importers remain subject to the statutory prohibition against the introduction or delivery for introduction into interstate commerce of adulterated food {Section 301(a) of the FD&C Act [21 U.S.C. 331(a)]}.
FSVPs for Importers of Food for Humans and Animals: Guidance for Industry, January 2018
FDA issued the final FSVP regulation for importers of food for humans and animals on November 27, 2015 (80 C.F.R. 74225). The FSVP regulation, codified in 21 C.F.R. 1.500 through 1.514, specifies the foods and importers to which the FSVP regulation applies and establishes requirements relating to:
• Use of qualified individuals to conduct FSVP activities
• Hazard Analysis
• Food and supplier evaluation
• Foreign supplier verification
• Corrective actions
• Record keeping
This guidance provides questions and answers to facilitate importers’ understanding of the FSVP requirements.
Considerations for Determining Whether a Measure Provides the Same Level of Public Health Protection as the Corresponding Requirement in 21 CFR part 112 or the Preventive Controls Requirements in part 117 or 507: Guidance for Industry, January 2018
This guidance describes FDA’s current thinking on considerations for determining whether a measure or procedure used in lieu of an FDA requirement in 21 C.F.R. Part 112, 117, or 507 provides the same level of public health protection as the corresponding FDA requirement.
Policy Regarding Certain Entities Subject to the Current Good Manufacturing Practice and Preventive Controls, Produce Safety, and/or FSVPs: Guidance for Industry, January 2018
The purpose of this document is to state the intent of FDA not to enforce certain regulatory requirements as they currently apply to certain entities and/or activities. This includes enforcement policy for importation of food contact substances under the FSVP regulation, enforcement policy for certain human food by-products for use as animal food that is further manufactured/processed, etc.
Further, FDA recently posted a document on its website that lists all importers that have been identified at entry in connection with the FSVP regulation. This posting is a statutory requirement under FSMA. The list provides all of the FSVP importer names that have been declared at entry.
Blockchain Technology and FSVP
Blockchain is a new technology tool for storing and sharing of information in open virtual network space. It has wide-ranging applications from finance to the food industry. It is certain that the technology will make the food supply chain more transparent than ever before by allowing users to look at the information associated with that food simultaneously. Further, it can greatly improve product traceability in the supply chain. Realizing the huge potential of this technology, big food corporations and retailers such as Nestlé, Unilever, and Walmart, and IT corporations such as IBM have invested huge resources to tap the full potential in the field of food safety. In addition to enhancing food product/ingredient traceability, it can reduce food waste by helping organizations target and withdraw only the specific batches of affected products during recalls.
Another potential application of blockchain technology is its use in food fraud. Food fraud is quite prevalent in many countries with the sole objective of economic gain. Unlike the common contaminants in ingredients, raw materials, and other food products that are known to occur, food fraud is difficult to detect. A well-known example of food fraud is the melamine incident in milk powders and pet foods that happened in 2008–2009. The need to enhance traceability and transparency in the food supply chain is overdue.
The FSVP rule requires importers to ensure their products are safe and ensure the integrity of the supply chain. The global food supply chain is complex, loaded with several unknown factors that an importer in the U.S cannot easily detect and control. While the regulatory requirements to ensure the integrity of the supply chain are clear, importers do not have effective tools to achieve these objectives. Blockchain technology is the best solution for this. Blockchain is nothing but a chain of blocks (records) arranged in a chronological order. Therefore, the possibility of altering records is greatly reduced.
Currently, the technology is in its infancy particularly as it applies to food safety. Nevertheless, it offers enormous potential for improved product traceability in the supply chain. Importers will be able to trace the full history of the products they are importing. It will be much easier to protect the integrity of the food supply chain and consequently prevent major foodborne illness as this new technology matures.
Challenges Ahead for FSVP Implementation
It is going to be a tough task for at least the very small importers to comply with the requirements within the specified timeline, knowing their limited technical capabilities and financial constraints. Implementation of the requirements of the FSVP rule is not a simple task. It requires a good understanding of the hazards associated with food products and their control measures. Although FDA has published guidance documents, importers are not yet fully prepared. The other major hurdle for very small importers is maintaining proper documentation and records. The food importer community needs to be more disciplined in this respect.
On the other hand, FDA may also face some challenges in enforcing the FSVP rule. The agency has to make sure the importer community understands the requirements of the rule; otherwise, there will be repeat failures on the part of importers. Kudos to FDA for making it clear that the initial phase of implementation of the rule is going to be an opportunity for importers to crawl before walking.
Dr. Ramakrishnan Nara is a technical adviser/consultant for the food, pharma, and dietary supplements industries. He can be contacted at nrkrish@rogers.com.
Reference
1. www.ers.usda.gov/data-products/us-food-imports/us-food-imports.





