Controlling Foreign Object Hazards in Food
The reliability of control systems should align with foreign object risk, and redundant controls should be implemented for high-risk hazards to increase the overall reliability

Foreign object (FO) control in the food industry requires more than just putting a metal detector at the end of the line. Comprehensive analyses of FO hazard pairs from ingredients, processes, and environments are required to avoid gaps in FO preventive controls that can cause injury, adulterated product, disruptions to the supply chain, damage to equipment, and damage to the company brand. Injuries to the mouth, throat, and/or digestive tract can occur when a person consumes a product containing a sharp FO. Traumatic tooth fractures occur when an unsuspecting consumer bites down on a hard FO. Plastic or rubber FO can cause choking, depending on the size, the product type, and the end user.
Those who have been injured by an FO in food may be eligible to file a personal injury lawsuit against the manufacturer of the food or the party responsible for preparing the food. Each U.S. state is different on rulings, although the Department of Agriculture's Food Safety and Inspection Service (USDA's FSIS) and the Food and Drug Administration (FDA) are consistent regarding the removal of adulterated products from commerce. Prevention of public health FO hazards in foods requires the implementation of redundant preventive controls, ongoing monitoring, and rapid corrective actions.
Years ago, I watched my dad break one of his front teeth on an FO while he was eating a hamburger at a well-known hotel restaurant. He was in Houston for an NFL golf tournament. Dad played football without a face mask most of his career, and his nose had been broken so many times he could press it flat to his face. All his front teeth were in perfect shape until he bit down onto an FO, however. When he showed me his broken tooth and the small, hard FO, we both laughed about it after the initial shock. What was not funny was the reluctance of the restaurant to take responsibility for the product they had served. My dad did not want to make a big deal about it, but I did.
I had previously been involved with broken tooth complaints. One of my roles over the years for various companies involved contacting customers that reported injuries to determine if their complaints were legitimate or economically motivated. Facts, knowledge of the processes, and forensic-type analysis led to valid conclusions.
For example, if the metal FO (3/16 × 1/2 inch) on the left in Figure 1 or the hard green plastic object on the right (3/8 × 1/2 inch) in Figure 1 was allegedly found inside a fine-grind product that had gone through a 1/16-inch plate, then the complaint was unlikely to be valid. However, if the FO was said to come from a product that was ground at ≥ 3/8 inch, then the complaint could be valid.
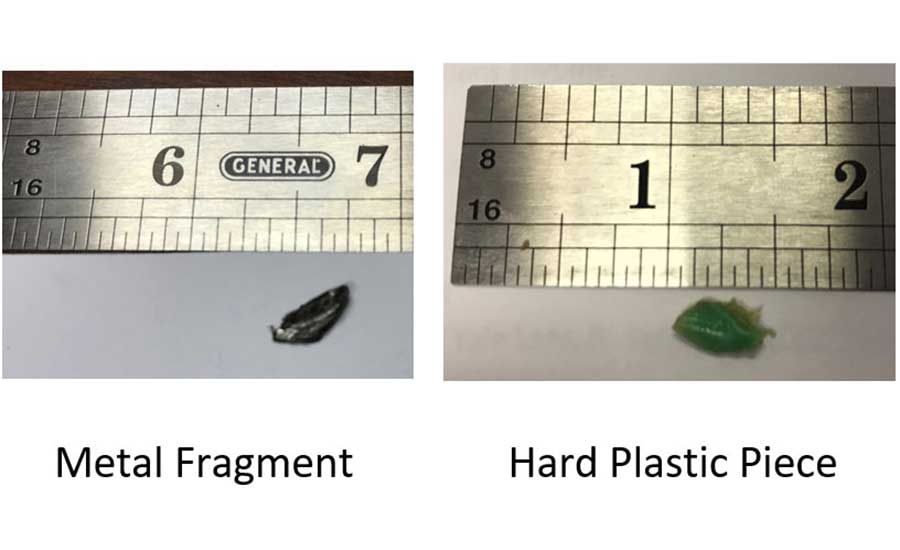
Over the years, I spoke with real people experiencing real injuries—some worse than others, but nonetheless, hardships caused by a product for which my company was responsible. In my previous experience, whenever the broken tooth complaint was real, my company paid the dental bills.
FO complaints from customers have triggered many product recalls and product safety alerts in the U.S. A plant or foodservice operation cannot afford to take the chance that one of their products leaves their control with an FO hazard in it. A review of the product-FO hazard pairs from FSIS and FDA Recalls, Withdrawals, and Public Health and Safety Alert data in 2021 and 2022 (Figure 2 and Table 1)1,2 reveal that most of the products were either multi-ingredient and/or further processed (as opposed to single-ingredient, intact). Data shows metal fragments (27 percent), hard/brittle plastic (22 percent), other plastic (thin, soft, not specified) (16 percent), other FOs (not specified) (12 percent) and glass (10 percent) to be the most prevalent FOs that reached the market in 2021 and 2022.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
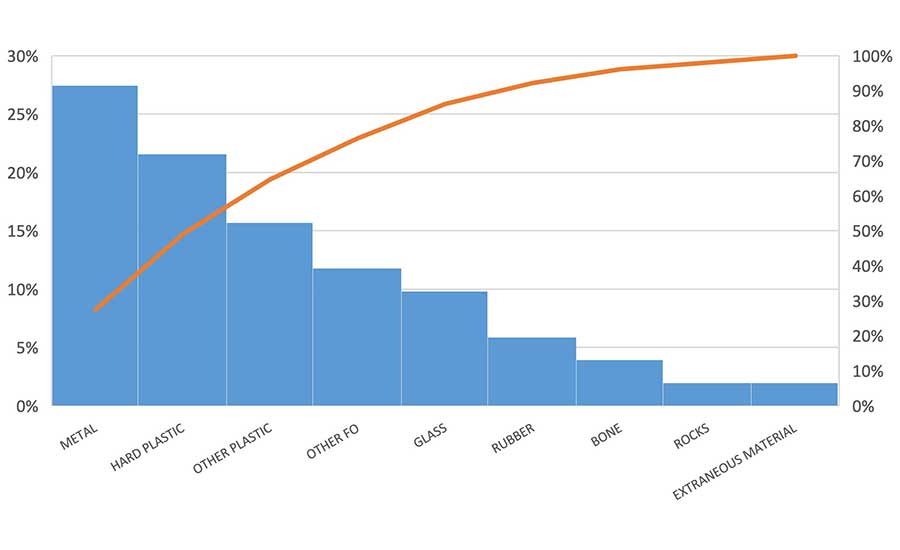
U.S. food companies involved with the approximately 50 FO recalls and alerts for adulterated foods in commerce over the two-year period (2021 and 2022) evidently had gaps in FO prevention controls.
What Does Regulatory Say about FOs in Food?
Under Federal Meat Inspection Act (FMIA)3 and Poultry Products Inspection Act (PPIA)4 regulations, the presence of foreign materials adulterates meat and poultry products, regardless of the physical characteristics of the foreign material5 (e.g., shape, size, hardness, etc.).
Ordinarily, bone particles in meat products are not considered foreign material because they are a natural part of the carcass. In a Federal Notice by FSIS, the agency wrote the following: "Objects inherent to a product are not 'foreign material,' however, the presence of these objects can render meat or poultry products adulterated."6 The FMIA and PPIA definition of adulterated states that, "…in case the substance is not an added substance, such article shall not be considered adulterated under this clause if the quantity of such substance in or on such article does not ordinarily render it injurious to health."7,8 However, if the size and amount of bone in a product would present a health hazard, then the product is adulterated.
FDA published a Compliance Guide for Foods, Adulteration Involving Hard or Sharp Foreign Objects in May 2005.9 Hard or sharp natural components of a food (e.g., bones in seafood, shell in nut products) are unlikely to cause injury because of awareness on the part of the consumer that the component is a natural and intrinsic part of a particular product. The exception occurs when the food(s) label represents that the hard or sharp component has been removed from the food, e.g., pitted olives. The presence of the naturally occurring hard or sharp object in those situations (e.g., pit fragments in pitted olives) is unexpected and may cause injury.
Recall Classifications: FSIS and FDA
USDA's FSIS10 and FDA11 assess the public health concern or hazard presented when a recall action is initiated for products adulterated with foreign material. USDA and FDA categorize recalls using the following systems:
- USDA Class 1: Reasonable probability that the use of the products will cause serious, adverse health consequences or death
- FDA Class 1: A situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death
- USDA Class 2: Remote probability of adverse health consequences from the use of the products
- FDA Class 2: A situation in which the use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote
- USDA Class 3: Products will not cause adverse health consequences
- FDA Class 3: A situation in which use of or exposure to a violative product is not likely to cause adverse health consequences.
Farm-to-Fork Risk Assessment for Controlling FO in Food
Incoming ingredients can be sources of FOs. Hazards inherent to ingredients are typically identified during the research and development of a new product or formula change.
FDA and USDA provide helpful resources for insight into ingredient FO pairs. These resources include Hazard Analysis and Risk-Based Preventive Controls for Human Food: Guidance for Industry, Chapter 3: Potential Hazards Associated with the Manufacturing, Processing, Packing, and Holding of Human Food,12 Fish and Fishery Products Hazards and Control Guidance,13 Juice HACCP Guidance,14 Meat and Poultry Hazards and Controls Guide,15 and USDA Guidebook for Developing HACCP Plans.16 Table 2 contains information from FDA's Quick Reference Guide for Common Sources of Physical Hazards.12
Product developers should collaborate with HACCP subject matter experts to evaluate the FO hazard risk of the supplier's ingredient(s). Once the ingredient and sub-ingredient food safety and non-food safety FO hazards are determined, the supplier preventive controls for the identified FO hazard pairs should be carefully studied before approval to purchase is granted.
New supplier performance history can be hard to obtain. Often, ingredient suppliers are represented by brokers, and it is more difficult to establish a direct relationship with the supplier(s). Whatever supplier approval methods are used, it is important to validate that the FO risks are reliably controlled by conducting heightened monitoring and data collection of the new ingredient(s) at receiving and during production to determine failure rates. Poor-performing suppliers should be disqualified.
The reliability of the control systems should align with the FO risk, where FO risk = probability + severity. Even with all the great engineering minds and new technology in the food industry, much of food manufacturing involves machinery and people. Both are prone to error. Therefore, redundant controls should be implemented for high-FO-risk hazards to increase the overall reliability, where reliability = 1 – the sum of the failure rates.
Implementation of Internal FO Prevention Programs
The same hazard pairs and reliability concepts apply inside a facility where performance or fitness for use is measured at each step in a process leading to the finished product. Internal processes and facility-related food safety FO hazards and non-food safety FOs should be thoroughly studied to determine the potential processing environment FO pairs. Once these pairs are understood, then the appropriate preventive controls for food processing can be built into the processing specifications for each piece of equipment in the facility.
The following flow model for developing FO hazard pairs is adapted from King and Bedale's Hazard Analysis and Risk-Based Preventive Controls.17
- What are the known FO hazards associated with these processes in this facility (e.g., equipment, ingredient, plant history)?
- Are any of these process-FO hazards already under preventive controls within the food manufacturing facility (e.g., 100 percent inspection, sifters, magnets, metal detectors, X-ray, vision systems)?
- List each process/facility-hazard pair that needs a preventive control specification. All the ingredient and processing/equipment hazard pairs related to FOs should be considered. Blueprints are useful to diagram out the processes, equipment, and possible process/facility-related hazards. The list of process/facility hazard pairs could look like the list in Table 3.
- Internal Preventive Controls for FOs. Assemble the HACCP team, review the hazard analysis, and implement controls in HACCP plans, prerequisite programs, work instructions, audit tools, and training materials.
In general, in evaluating the potential for FO hazards in food products, it is advisable to study each facility environment and individual piece of processing equipment. Physical hazards can be readily classified as facility-related or process-related. For example, wire or welding slag from maintenance off-shift repairs are facility-related FO hazards. Production equipment that dices or grinds, a batching process involving emptying ingredient bags, scrapper blade assemblies in a cook kettle, or a combo dumper with loose parts that could fall into the product zone are process-related hazards.
After the hazards and preliminary FO controls are decided for each piece of equipment, employee education materials are developed. Work instructions come in various forms (digital, or in production programs or preventive maintenance programs). Routine observation and interview audits verify and reinforce their importance to the workforce.
The SOP for how hazard pairs are assessed can be included in a Food Safety Management of Change program. This program might already include precautionary procedures for sanitary design of equipment, modification and repair of equipment, construction of equipment, etc. The system does not require elaboration; the concept and daily awareness are what matters. Assessments should be ongoing so that chosen actions are subject to review.
Product Zone
The product zone is a three-dimensional zone around and above exposed product or food contact surfaces. Monitoring the potential for FO introduction from the processing environment is an ongoing task of all departments before, during, and after production. A good practice is to verify that the product zones are free of potential hazards at pre-operation and take corrective action, as needed, before startup. Damage can occur during off-shifts when guards are removed for maintenance or during sanitation (i.e., chipping a Lexan safety guard or not replacing a bolt, cracking an overhead light cover not in the product zone, etc.). Damage on off-shifts, when no product is involved, does not pose a food safety risk of adulterated product; however, it is difficult to prove that the damage did not occur during production unless there are records (Figure 3).

Ongoing verification of the product zone and FO preventive controls builds workforce awareness. Glass and brittle plastic audits are useful, as are maps showing the locations where these hazards have not been eliminated. However, catching and isolating a potential FO is best accomplished with ongoing, daily focus on the product zone FO hazard potential. Accounting for all ingredient bags, checks to verify that wooden combo pallets have been stretch-wrapped to prevent wood splinters or loose pallet nails from accidently being dumped into a mixer, and grinder plate/blade checks during operation are examples of verifying preventive controls.
Understanding the importance of the product zone is essential for all departments and the entire workforce. Training to understand these concepts is critical to ensuring food safety.
Finished Product Metal Detection
Metal detector operators and their supervisors have significant responsibility when it comes to handling the rejects. Confirmed rejects must be dissected and evaluated to find the object that caused the reject and remove it for further examination and follow-up action, if necessary. Sensitivity of detection systems is often set on the edge of the system's capability without creating excessive false rejects. Nonetheless, false rejects are commonplace, and the workforce can be lulled into not taking the rejects seriously if too many occur. Optimization of metal detector systems varies from plant to plant and process to process. Companies establish programs whereby each occurrence is reviewed, plus an escalation if a specific number of occurrences are observed in an established period of time. Pre-established corrective actions for FO events include holding the finished product until the plant investigation is complete. It should be noted that an FO contamination event is not the same as random/"tramp metal" rejects. Tramp metal is a term used for a very small, isolated piece of metal that has no known source.
Contrary to some beliefs, control point and critical control point systems are not in place to screen product or remove the hazard(s). They indicate if a failure upstream requires corrective action. It if does, the respective product [work in process (WIP) or finished] should be evaluated under a new set of conditions before moving to the next step in the process or being released to commerce.
A Roadmap to Foreign Object Response during Production
FO incidents are routine in food manufacturing plants. A well-trained plant team can minimize the disruption to production and costs and ensure regulatory compliance when an FO incident occurs.
FOs discovered during production range from small, isolated incidents that are easily remedied to widespread contamination that result in major product losses. One of the keys to preventing widespread contamination is to catch the contamination early. One of the keys to maintaining control and staying out of regulatory trouble is to demonstrate control by putting product on hold before a regulatory agency determines it needs to act. Finally, it is imperative to have records to prove that the establishment controlled the situation and made an appropriate disposition.
The following is a basic guide for FO discoveries during production where no suspect product has left the control of the establishment. USDA provides a guideline for industry on how to respond to customer complaints of meat and poultry products contaminated with foreign material.18
1. Prevent Loss of Evidence
Depending on the process, when an FO is discovered by visual observation or by hearing an unusual noise while processing, professional food handlers often do a controlled stop and lockout/tagout, to investigate and report to their supervisor. As soon as the supervisor determines there could be product involved, then the next-level managers need to become involved.
FO incidents are good opportunities for the workforce to see the leaders walk the talk about following company procedures like professionals. All efforts should be made to isolate the suspect product and prevent the FO(s) from getting lost. The goal is to account for all the missing pieces and develop a time line of when the contamination started. When it stopped should also be determined. Discovery examples include finding an FO when prepping ingredients for batching; hearing a loud noise from processing equipment; metal detector rejects; finding an FO inside equipment during production; or finding damaged equipment during operation, when equipment is torn down at the end of production, or during the sanitation shift. The importance of securing all of the evidence comes into play during the investigation when the extent of the problem is being assessed.
2. Identify and Control All Suspect Product and Notify Plant Management
Utilize a lot control system (preferably digitized) to trace forward and trace back, as needed. Place suspect product on computer hold and/or physically tag or tape off suspect product. Depending on the circumstances, it could be prudent to conduct a mock recall while investigating. This is a plant team effort. Plant management needs to be notified early to be made part of the assessment. Depending on the severity of the situation, maintenance and engineering resources may need to be assigned to replace or repair equipment parts, and production scheduling may need to be changed. Controlling product at this stage serves two purposes: 1) Preventing the creation of additional suspect product, and 2) providing a record showing that the establishment has control until the assessment is complete. When the establishment takes control, this prevents the government from taking agency action.19,20,21
Suppose a mixer operator has unloaded three vats of WIP, and as the operator unloads the fourth and final vat, a torn piece of plastic that looks like part of a spice bag is discovered wrapped around one of the ribbon shafts. Quick action needs to be taken to stop one of the previous vats from being used at the next processing step. The objective is to isolate all suspect WIP that could have plastic in it. The suspect vats of product should be identified with hold tags, and the mixer should be locked out and the area taped off so that no vats will be accidentally picked up and moved, and so that no evidence is lost. If the plastic bag can be 100 percent reconstructed by finding all the pieces, then the WIP can be saved. If not, then a determination must be made as to when the spice bag accidently got in the mixer. Was it the last mix, or the one before that? This example also points out the benefit of inspecting and documenting the condition of equipment between process batches (e.g., free of FOs) between mixes.
Suppose a grinder operator hears an unusual noise but does not act. Some time later, the grinder locks up. The consequences of not stopping could cause additional damage to the equipment and more product contamination. The entire ground product from the initial noise is suspect. The grinder and the product inside the grinder must be isolated and all the evidence secured for the investigation—the same as the plastic in the mixer. The goal is to find the logical starting and stopping points. This is the benefit of establishing standard procedures for documenting the time and FO conditions of food contact equipment and product zones before, during, and after production.
3. Assess Problem and Develop an Action Plan
Assessing the situation and developing an action plan is where preventing loss of evidence in the previous steps comes in. If the plant team reacts appropriately and can account for all the spice bag pieces, then the product can be released.
If, on the other hand, the mixer operator simply removes the torn bag without telling anyone or controlling the WIP, and at subsequent steps in the process many small plastic pieces were later discovered, then the cost to the company will be significantly higher. More product will be involved, and labor dollars have been spent processing plastic-contaminated product that must be discarded. An entire day's production could be lost.
The same holds true for the grinder example. There is a good chance that all the FO contamination can be minimized if all suspect product is isolated and if the evidence is secured. However, if the grinder is disassembled and rinsed out with water for maintenance inspection, then some of the little pieces of the broken blade will be lost down the drain, and the part cannot be reconstructed. If all the missing pieces cannot be accounted for, then all product associated with the materials from the failed process are "adulterated" until proven otherwise, and must be discarded.
4. Implement Corrective Action22 and Keep Good Records23
Implementing the corrective action plan that plant management has agreed to is a team effort. Professional, cross-functional processing teams including members from production, maintenance, and FSQA can implement corrective action plans for FO contamination events as efficiently as pit crews in the Indy 500.
Sometimes, the small amount of product involved is not worth the cost of salvage efforts. In cases where the investigation shows the contamination is in one mix or one vat, plant management may choose to discard that product and do a complete washdown and inspection of all equipment involved. In other cases — for example, a missing filler O ring on a specific line — plant management may decide to "recondition"24 the product by sorting through the vats of filled product or WIP to find or reconstruct the O ring, torn plastic, or metal blade using a method that has been proven to be effective.
The scale illustration shown in Figure 4 is an example of how to prove that all broken blade pieces are recovered (equal weights = 100 percent recovery). In this example, suppose that an FO did not cause the blade to break; rather, frozen raw materials that were not tempered properly before grinding, plus a lower-cost blade from a new supplier, were the cause of the broken blade.

5. Notify In-House Government Inspector
The process of notifying an in-house inspector applies primarily to USDA-regulated plants. It should be noted that contamination events identified and 100 percent controlled within an establishment do not require notification of the district office (DO). The DO should be notified if a confirmed food safety FO hazard was identified in commerce via a customer complaint. Hazardous FOs in FDA product in commerce would trigger a report to the Reportable Food Registry.25
Notifying the in-house government inspector is just that—a courtesy notification from plant management with the facts and demonstration of control. It is not to ask the inspector how to handle the situation. Informing the inspector about what happened and how the establishment took control of the deviation, along with providing the facts and the supporting documents, heads off any misinformation that may result from exaggerated rumors among plant personnel. It is a show of respect and transparency that builds trust.
6. Reassessment/Reanalysis of Food Safety Plan
Suppose the metal detector or X-ray at the end of the line is the critical control point (CCP). If an FO discovery occurs at an upstream control point (CP)—e.g., an unusual amount of tramp metal on a rare earth magnet, pieces of metal fragments in sifter tailings, a partial spice bag in the mixer, or a broken grinder blade discovered during a plate check—then a reassessment of the food safety plan should take place. This reassessment will help in understanding what caused the FO contamination and allow the establishment to decide if changes should be made to improve the processes, even if these steps are CPs and not CCPs.
USDA-regulated product requirements for reassessing the HACCP plan are spelled out26 in the HACCP regulation and corresponding directive,27 which deal witha food safety CCP reassessment. At a minimum, the establishment should address the contamination in its hazard analysis,28 provide documentation that demonstrates that the contamination is not a food safety hazard, and indicate whether it controls foreign material contamination in its sanitation standard operating procedures (SSOPs) or in a prerequisite program. The corrective action and reassessment practices are worth doing, regardless of whether they fall under HACCP or SSOPs.
FDA-regulated product requirements are to perform a reanalysis29 whenever new information about potential hazards associated with the food are brought to light; whenever appropriate after an unanticipated food safety problem;30 and whenever a preventive control, a combination of preventive controls, or the food safety plan is found to be ineffective. The reanalysis may result in additional preventive controls, which may need to be validated.
A reassessment/corrective action record signed by a HACCP-trained individual can be easily accomplished by including the following information on one or two pages:
- Reason for the reassessment/reanalysis: Describe what happened, and include how quickly it was under plant control.
- Describe what corrective actions were taken.
- Disposition of product: Identification and quantify disposed and where disposed. If product was destroyed, describe and have proof.
- Describe if the HACCP plan was changed (keep it simple—yes, no, or N/A).
- Reasons/justification for changing or not changing the HACCP plan: Explain logic and provide support for deciding if the hazard was a potential food safety or non-food safety adulterant. It is rare that the HACCP plan is changed (although it can happen), and it is an important regulatory question that must be answered each time. This record is focused on corrective actions that ensure no adulterated product from that specific incident entered commerce. Long-term improvement plans with preventive measures are best recorded somewhere else, and not on this record for the regulatory agencies.
In addition to driving continuous improvement, documenting the reassessment and corrective actions ensures that the minimum regulatory requirements are met and avoids the chance of a noncompliance report being issued for not reassessing and/or documenting corrective actions after the event.
7. After-Action Review and Continuous FO Prevention Improvement
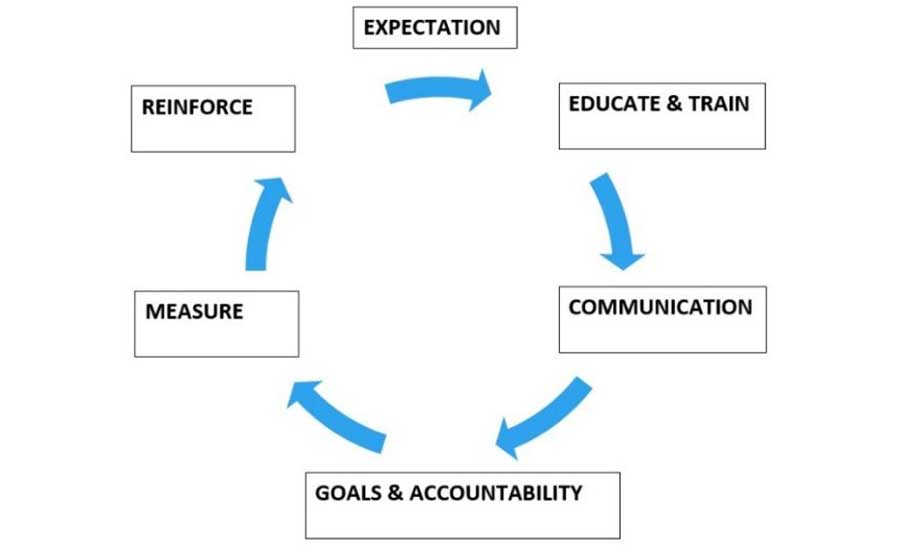
Leadership teams often meet, after the dust has settled, to follow up on corrective action plans and to discuss what went well and what could be improved. Once the corrective actions are closed out, this is the time to brainstorm and identify as a group what preventive control(s) failed or were missing. The leadership team considers the seriousness of the FO harm to public health and the cost/benefit to the business, with consideration of positive and negative consequences of improving preventive controls. In addition, the team considers the plant food safety culture implications of both action and inaction. These meetings can be a continuous improvement opportunity where leadership can move the culture in a right, wrong, or an indifferent direction. They are held in conjunction with routine production gatherings, separate group meetings, or one-on-one, depending on the case. After-action reviews are in line with the Frank Yiannas behavior-based food safety management system continuous improvement model31 (Figure 5).
Leadership's Role in Preventing and Managing FO Contamination
Leadership's role regarding FO prevention is to set the expectations, lead by example, and reinforce workforce behaviors that are aligned with company standards.
FO incidents during production cause conflicts of interest for production. Supervisor performance is measured on how well throughput, yield, labor, and efficiencies are maximized each day to meet production schedules. FO discoveries or incidents are disruptive. They can have a negative impact on production goals. It is understandable for a production supervisor to get upset when an FO is discovered.
However, each incident is also an opportunity for leaders to positively reinforce workforce behavior.
One of the biggest influences on food safety culture around FOs is when the workforce sees supervisors and managers consistently abiding by the company standards. Managers help reinforce good practices when the workforce sees them show up on the floor during an FO incident. For example, workforce behavior is positively influenced when supervisors professionally respond to FO incidents, or when the workforce learns that the company has stopped doing business with a supplier that has been found to have repeat FO issues.
Another effective positive influence is the enforcement of progressive discipline when an employee knowingly disregards established company standards for FO prevention. On the other hand, workforce behavior is negatively influenced when management is seen not responding seriously when an FO is discovered, not following SOPs, or doing nothing to fix a known source of FOs.
Conclusion
An FO can be accidentally introduced at many points along the farm-to-fork supply chain. Ingredients may pass through multiple high-speed, complex food manufacturing plant environments and processes before they are combined with multiple other supplier ingredients and then processed or prepared in the final environment. USDA and FDA provide U.S. food processors with an excellent framework for FO prevention. Ingredient suppliers, ingredient processors, warehouses, finished goods manufacturers, and retail establishments can and do minimize the public health risk and disruptive impact of FOs by understanding the farm-to-fork hazard pairs, implementing reliable preventive controls, and responding to FO discoveries during production with trained workforce teams that follow company-established best practices.
If the reader is interested to know, the hotel restaurant where my dad broke his tooth while eating a hamburger finally reimbursed him for the tooth extraction and implant—a year and a half later. The dental bill was about $4,000. I have no idea what the hotel's attorney fees were.
References
- USDA Food Safety Inspection Service (FSIS). "Recalls & Public Health Alerts." https://www.fsis.usda.gov/recalls.
- U.S. Food and Drug Administration (FDA). "Recalls, Market Withdrawals, & Safety Alerts." https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts.
- USDA FSIS. "Federal Meat Inspection Act." https://www.fsis.usda.gov/policy/food-safety-acts/federal-meat-inspection-act.
- USDA FSIS. "Poultry Products Inspection Act." https://www.fsis.usda.gov/policy/food-safety-acts/poultry-products-inspection-act.
- USDA FSIS. "Product Contamination." https://www.fsis.usda.gov/taxonomy/term/16.
- USDA FSIS. "Availability of FSIS Guideline for Industry Response to Customer Complaints." December 21, 2020. https://www.federalregister.gov/documents/2020/12/21/2020-28112/availability-of-fsis-guideline-for-industry-response-to-customer-complaints.
- U.S. Code. "Title 21, Subchapter 1: Inspection Requirements: Adulteration and Misbranding §601: Definitions." https://www.govinfo.gov/content/pkg/USCODE-2021-title21/pdf/USCODE-2021-title21-chap12-subchapI-sec601.pdf.
- U.S. Code. "Title 21 §453: Definitions." https://www.govinfo.gov/content/pkg/USCODE-2021-title21/pdf/USCODE-2021-title21-chap10-sec453.pdf.
- FDA. "CPG Sec 555.425: Foods, Adulteration Involving Hard or Sharp Foreign Objects." Compliance Policy Guide. May 2005. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-555425-foods-adulteration-involving-hard-or-sharp-foreign-objects.
- USDA FSIS. "Understanding FSIS Food Recalls." October 16, 2015. https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/understanding-fsis-food-recalls.
- U.S. Code. "Title 21, Chapter 1, Subchapter A, Part 7, Subpart A, § 7.3: Definitions." https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-7/subpart-A/section-7.3.
- FDA. Hazard Analysis and Risk-Based Preventive Controls for Human Food: Draft Guidance for Industry. "Chapter 3: Potential Hazards Associated with the Manufacturing, Processing, Packing, and Holding of Human Food." https://www.fda.gov/media/99558/download and https://www.fda.gov/media/99581/download.
- FDA. Fish and Fishery Products Hazards and Controls Guidance. June 2022. https://www.fda.gov/media/80637/download.
- FDA. Juice Hazard Analysis Critical Control Point Hazards and Controls Guidance. March 2004. First Edition. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first#iii.
- USDA FSIS. Meat and Poultry Hazards and Controls Guide. March 2018 https://www.fsis.usda.gov/sites/default/files/import/Meat_and_Poultry_Hazards_Controls_Guide_10042005.pdf.
- USDA FSIS. Guidebook for the Preparation of HACCP Plans. https://www.fsis.usda.gov/sites/default/files/media_file/2021-01/Guidebook-for-the-Preparation-of-HACCP-Plans.pdf.
- King, Hal and Wendy Bedale. Hazard Analysis and Risk-Based Preventive Controls. Elsevier, October 2, 2017. https://www.elsevier.com/books/hazard-analysis-and-risk-based-preventive-controls/king/978-0-12-809475-4.
- USDA FSIS. "Availability of FSIS Guideline for Industry Response to Customer Complaints." December 21, 2020. https://www.federalregister.gov/documents/2020/12/21/2020-28112/availability-of-fsis-guideline-for-industry-response-to-customer-complaints.
- U.S. Code. "Title 9, Chapter 3, Subchapter E, Part 500: Rules of Practice." https://www.ecfr.gov/current/title-9/chapter-III/subchapter-E/part-500.
- U.S. Code. "Title 9 Chapter 3, Subchapter A, Subpart 318.2: Reinspection, Retention, and Disposal of Meat and Poultry Products at Official Establishments." https://www.ecfr.gov/current/title-9/chapter-III/subchapter-A/part-318/subpart-A/section-318.2.
- U.S. Code. "Title 21, Subchapter 1: Inspection Requirements: Adulteration and Misbranding §601: Inspection and Labeling of Meat Food Products." https://www.govinfo.gov/content/pkg/USCODE-2014-title21/html/USCODE-2014-title21-chap12-subchapI-sec606.htm.
- U.S. Code. "Title 9, Chapter 3, Subchapter E, Part 417: Corrective Actions." https://www.ecfr.gov/current/title-9/chapter-III/subchapter-E/part-417/section-417.3#p-417.3(b)(4).
- U.S. Code. "Title 9, Chapter 3, Subchapter E, Part 417: Subpart 417.5: Records." https://www.ecfr.gov/current/title-9/chapter-III/subchapter-E/part-417/section-417.5#p-417.5(c).
- U.S. Code. "Title 21, Chapter 1, Subchapter B, Part 117, Subpart B, § 117.80: Process Controls." https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-117/subpart-B/section-117.80.
- FDA. "Reportable Food Registry for Industry." https://www.fda.gov/food/compliance-enforcement-food/reportable-food-registry-industry.
- U.S. Code. "Title 9, Chapter 3, Subchapter E, Part 417, § 417.4: Validation, Verification, Reassessment." https://www.ecfr.gov/current/title-9/chapter-III/subchapter-E/part-417/section-417.4.
- USDA FSIS. "Presence of Foreign Material in Meat or Poultry Products—Revision 3." FSIS Directive 7310.5. https://www.fsis.usda.gov/policy/fsis-directives/7310.5.
- U.S. Code. "Title 9, Chapter 3, Subchapter E, Part 417, § 417.2: Hazard Analysis and HACCP Plan." https://www.ecfr.gov/current/title-9/chapter-III/subchapter-E/part-417/section-417.2.
- U.S. Code. "Title 21, Chapter 1, Subchapter B, Part 117, Subpart C, § 117.170: Reanalysis." https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-117/subpart-C/section-117.170.
- U.S. Code. "Title 21, Chapter 1, Subchapter B, Part 117, Subpart C, § 117.150: Corrective Actions and Corrections." https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-117/subpart-C/section-117.150#p-117.150(b).
- Yiannas, Frank. Food Safety Culture—Creating a Behavior-Based Food Safety Management System. Springer, December 10, 2008.
Yale Lary, Jr. is an Expert Advisor at Active Food Safety. Yale completed his graduate studies at Texas A&M University, where his research was funded by USDA through the Animal Science Department. He has years of hands-on experiences across a wide variety of supply chains, food types, manufacturing processes, and cultures. He has expert knowledge of food microbiology, livestock and poultry production, biosecurity, and animal welfare. Yale has been actively associated with the International Association for Food Protection, the Food Research Institute, and the Professional Animal Auditor Certification Organization throughout his career. He has held leadership positions for several major corporations and midsized companies. His diverse work experiences combined with his passion for learning and working with teams to find creative solutions enable him to identify risks, troubleshoot, collaborate with teams, improve processes, train associates, and implement sustainable programs that add value and improve food safety.







