GFSI’s Role in Harmonizing Food Safety Standards

The Global Food Safety Initiative (GFSI) emerged in 2000 to address the need to ensure food safety in Europe. Traditionally, there have been a number of differences between the European and U.S. food retailing chains. One of the most noted differences is that, in Europe, private-label foods have consistently grown in sales. Between 1975 and 1997, for example, the average private-label product doubled in market share in European retail outlets.1 In 2001, private-label brands consisted of 42% of the sales in Tesco and 34% of the sales in Safeway (Table 1). Market surveys estimated that private labels accounted for 40% of European retailer sales in 2005.[1]
Thus, the need arose for retailers to protect their private brands. Previously, major retailers either had internal audit standards and auditors or required specific third-party audits, usually at the supplier’s expense. While they were all trying to achieve the same goal, the production of safe food, the requirements were all different, which meant processors had to grapple with multiple auditing standards and audit types. There were simply too many variables and standards.
One food manufacturing company stated that it supplied 25 retailers, which meant it submitted to three customer corporate audits and four different third-party audits—hence, seven different standards that did not compare “apples to apples.” This particular company was also regulated by the state, which added four more audits to the mix. When the facility’s quality manager was asked by an auditor, “Do you conduct monthly inspections at your facility?” the quality manager replied, “Why do I need to do monthly inspections when I’m already audited every month by a customer or third-party auditors?” Consolidating the number of audit standards to the GFSI’s current four is a step in the right direction, provided that all retailers embrace the GFSI standards.
Development of Food Safety Standards
In 1998, the British Retail Consortium (BRC) developed and introduced the BRC Global Standard for Food Safety to be used to evaluate manufacturers of retailers’ own brand of food products. This standard is now in its fifth edition, which was published on January 4, 2008, and has been translated into 10 languages: Chinese (Simplified), Czech, Dutch, French, German, Norwegian, Polish, Spanish, Swedish and Italian. The BRC Global Standard for Food Safety has evolved into a globally accepted standard used not just to assess retailer suppliers but also as a framework upon which many companies have based their supplier assessment programs and manufacture of products. Following the success of the Global Standard – Food, the BRC published the first issue of the Packaging Standard in 2002, followed by the Consumer Products Standard in August 2003 and finally by the BRC Global Standard – Storage and Distribution in August 2006. Each standard is regularly reviewed and is fully revised and updated at least every three years after consultation with a wide range of stakeholders. These standards are available on BRC’s Web site at www.brc.org.uk/. The major elements of the BRC Global Standard for Food Safety are provided in Supplementary Table 1.
Supplementary Table 1:
The Major Elements of BRC—Global Food Safety Standard Edition 5
Senior management commitment and continuous improvement
Food safety plan (the five preliminary steps and the seven principles of HACCP)
Food safety and quality management system
Food safety and quality policy
Food safety and quality manual
Organizational structure, responsibilities and management authority
Contract review and customer focus
Internal audits
Purchasing — supplier approval and performance monitoring
General documentation requirements
Corrective and preventative action
Traceability
Complaint handling
Management incidents, product withdrawal, and product recall
Site standards
External standards
Security
Internal site standards
Utilities
Equipment
Maintenance
Staff facilities
Chemical and product contamination control
Housekeeping and hygiene
Water/waste disposal
Pest control
Storage and transport
Product control
Product design/development
Handling requirements for specific materials — materials containing allergens and identify preserved materials
Foreign body detection
Product packaging
Product inspection and laboratory testing
Control of non-conforming product
Product release
Process control
Control of operations
Quality — weight, volume and number control
Calibration and control of measuring and monitoring devices
Personnel
Training
Access and movement of personnel
Personal hygiene
Medical screening
Protective clothing
Source: BRC. 2008. Global standard for food safety, Issue 5. British Retail Consortium, London UK.
The International Food Standard (IFS) was first published in 2003 by the German Retail Union food retailers (Hauptverband des Deutschen Einzelhandels or HDE). In 2003, French retailers and wholesalers from France’s Federation of Commercial and Distribution Companies (FDC) joined the IFS Working Group and contributed to the development of the IFS standard’s fifth edition, which was published August 5, 2007. The IFS standard is available at www.ifs-certification.com. Its major elements are described in Supplementary Table 2.
Supplementary Table 2:
The Major Elements of IFS — International Food Standard Edition 5
Senior management responsibility
Corporate policy/corporate principles
Corporate structure
Customer focus
Management review
Quality management system
HACCP - The five preliminary steps and the seven principles of HACCP
HACCP system
Assemble HACCP team
HACCP analysis
Documentation requirements
Record keeping
Resource management
Human resources management
Human resources
Personal hygiene
Protective clothing for personnel, contractors, and visitors
Procedures applicable to infectious diseases
Training
Sanitary facilities, equipment for personnel hygiene and staff facilities
Production process
Contract review
Product specifications
Product development
Purchasing
Product packaging
Factory environment standards
Choice of location
Exteriors
Plant layout and process flows
Building and facilities
Constructional requirements
Walls and partition walls
Floors
Ceilings/overheads
Windows and other openings
Doors
Lighting
Air conditioning (ventilation)
(Drinking) water supply
Housekeeping and hygiene
Waste and waste disposal
Risk of foreign bodies, metal, broken glass and wood
Pest monitoring/pest control
Receipt of goods and storage
Transport
Maintenance and repair
Equipment
Process validation
Traceability including GMOs and allergens
Genetically modified organisms (GMOs)
Allergens and specific conditions or production
Measurements, analysis improvement
Internal audits
Site factory inspections
Process control
Calibration and checking of measuring and monitoring devices
Quantity checking(quantity control/filling quantities)
Product analysis
Product quarantine and product release
Management of complaints from authorities and customers
Management of incidents, product withdrawal, product recall
Management of non-conforming products
Corrective actions
Source: IFS. 2007. International food standard – Standard for auditing retailer and wholesaler branded food products version 5. HDE Trade Services GmbH, Berlin, Germany.
The Safe Quality Food (SQF) 2000 Code provides a food safety and quality management certification program that is tailored to the needs of the food processor. The code was developed in Australia in 1994, and pilot programs were implemented to ensure its applicability to the food industry. It was prepared with the assistance of experts in quality management, food safety, food regulation, food processing, agriculture production systems, food retailing, food distribution and the Hazard Analysis Critical Control Point (HACCP) guidelines. The Food Marketing Institute acquired the rights to the SQF program in August 2003 and has established the SQF Institute (SQFI) division to manage the program. The SQF 2000 Code Level 2 is recognized by the GFSI as a standard that meets its benchmark requirements. The SQF 2000 Code has evolved with time and the sixth edition, published in August 2008, is posted on the SQFI Web site at sqfi.com. The SQF 2000 Code is available in both English and Spanish; its major elements are described in Supplementary Table 3.
Table 3:
The Major Elements of SQF 2000 Code —
A HACCP-based Supplier Assurance Code for the Food Industry Edition 6
SQF 2000 system requirements
Commitment
Management policy
Management responsibility
Food safety and quality management system
Management review
Complaint management
Business continuity planning
Documentation control and records
Documentation control
Records
Specifications and product development
Product development and realization
Raw materials
Packaging
Contract service providers
Contract manufacturers
Finished product
Attaining food safety
Food legislation (regulations)
Food safety fundamentals
Food safety plan
Food quality plan
Incoming goods and services
Corrective and preventative action
Non-conforming product or equipment
Product rework
Product release
Stock rotation
Verification
Responsibility, frequency, and methods
Validation
Verification of monitoring activities
Product sampling, inspection, and analysis
Internal audits
Verification schedule
Product identification, trace, withdrawal, and recall
Product identification
Product trace
Product withdrawal and recall
Site security
Food defense
Identity-preserved foods
General requirements
Food safety fundamentals — building and equipment design and construction
Site requirements and approval
Premise location
Construction and operational approval
Food handling areas
Materials and surfaces
Floors, drains, and waste traps
Walls, partitions, doors, and ceilings
Stairs, catwalks, and platforms
Lighting and light fittings
Inspection area
Dust-, fly- and vermin-proofing
Ventilation
Equipment, utensils, and protective clothing
Cleaning of equipment, utensils and protective clothing
Hand-washing facilities
Protective clothing racks
Vehicles
Water and ice supply
Water supply
Water delivery
Ice supply
Water treatment
Storage facilities
Cold storage, freezing, and chilling of foods
Storage and dry ingredient storage and shelf-stable packaged goods
Storage and packaging
Storage of equipment and receptacles
Storage of hazardous chemicals and toxic substances
Alternative storage and handling of goods
Separation of functions
Process flow
Receipt of raw materials
Thawing of product
High-risk processes
Other processes
On-site laboratories
Location
Staff amenities
General
Change rooms
Showers
Laundry
Sanitary facilities
Lunchrooms
First aid facilities
Access to first aid
Waste disposal
Dry and liquid waste disposal
Exterior
Grounds and roadways
Food safety fundamentals prerequisite programs
Personnel practices
Personnel
Clothing jewelry and personal effects
Visitors
Personnel processing practices
Staff engaged in food handling and processing operations
Training of personnel
Training requirements
Training program
Instructions
HACCP training requirements
Language
Refresher training
Training skills register
Calibration of equipment
Calibration methods
Calibration standards
Calibration schedule
Records
Management of pests and vermin
Requirements
Pest and vermin control program
Using pest-control chemicals
Pest-control contractors
Disposal of unused pest-control chemicals
Premises and equipment maintenance
Maintenance program
Instruction to maintenance personnel and contractors
Maintenance schedule
Equipment lubrication and paints
Cleaning and sanitation
Cleaning and sanitation program
Evaluating the effectiveness of cleaning
Purchasing storage and use of detergents and sanitizers
Disposal of unused detergents and sanitizers
Monitoring water microbiology and quality
Standard
Water Treatment
Analysis
Control of physical contamination
Foreign material
Detection of foreign objects
Managing foreign matter contamination incidents
Supplier approval
Selecting approved suppliers
Approved supplier program
Monitoring approved suppliers
Register
Records
Transport and delivery
Transport, loading, and unloading practices
Loading
Transport
Unloading
Waste management and disposal
Dry, wet and liquid waste
Removal from food handling and processing areas
Maintaining waste removal equipment and areas
Monitoring waste removal
Allergen control
Allergen control program
Risk analysis
Receiving and storage raw materials
Storing product containing allergy-causing agents
Sanitation of processing area and equipment
Batch identification and trace
Re-working product containing allergy-causing agents
Requirements for food contained in hermetically sealed rigid, flexible or semi-rigid containers
Canning operations
Canning equipment
Establishing the scheduled process
Thermal processing
Seam and seal integrity
Quality assurance
Source: www.sqfi.com.
The SQF 1000 Code provides a food safety and quality management certification program for the primary producer. A draft of that code’s fifth edition was available for comment by May 1, 2009. On February 4, 2009, GLOBALGAP and SQFI announced that the SQF 1000 standard will be benchmarked to the GLOBALGAP standard. Benchmarking by GLOBALGAP means that SQF will be able to offer produce growers a food safety certification that is recognized by both GFSI and GLOBALGAP. As a result, a primary producer will be able to receive both SQF and GLOBALGAP certification at the same time in a single audit. The SQF Codes are reviewed every three years by the SQFI Technical Advisory Council.
In 2004, the Foundation of Food Safety Systems (SCV) was founded by the National Board of Experts in the Netherlands. The SCV maintains the Dutch certification scheme for a standard that is known as Dutch HACCP. For all practical purposes, Dutch HACCP has not emerged as a major player in the U.S. market, so this standard’s major elements are not described here. However, the standard is available at www.foodsafetymanagement.info.
These private entities began to develop food safety standards for certification, bringing increased costs to European food retailers since a food processor may have sold product to several retailers, each wanting products to be certified to a different standard.
ISO 22000: Bringing Harmony to the Standards
In 2001, Danish Standards requested that ISO undertake the development of an international food safety management standard. By this time, there were not only a large number of private standards but a number of countries—including Ireland, the Netherlands, Germany, Denmark, Australia and the United States—also had their own national food safety standards. Thus, the ISO sought to harmonize and publish an international food safety standard. In 2005, the ISO working group accomplished its objective and published ISO 22000.
ISO standards are developed using a transparent process that is designed to encourage input from all of the standard’s stakeholders. This is done by having both a national Technical Advisory Group (TAG) and an international standards working group. Sometimes a TAG is called a Mirror Group. The TAG is open to all professionals with the appropriate credentials who want to contribute to the standards development or review process. At the national level, each TAG develops a consensus position that is taken to the international committee by the national delegation.
ISO 22000 has a structure similar to ISO 9001:2000 and other ISO management system standards, such as ISO 9001 (quality management), ISO 14001 (environmental management) and OHSAS 18001 (occupational health and safety management). This allows the development of multiple management systems using a similar structure and the potential sharing of general management system procedures.
ISO 22000 made several significant contributions to the understanding of food safety management systems. First, it strengthened the need for food safety communications both internally and externally. Second, it introduced requirements such as emergency preparedness and formal updating of the food safety management system. Third, ISO 22000 was the first standard to separate validation from verification. This brought the standard in alignment with the draft version (and eventually, the final version) of the Codex standard.[2] Finally, the requirements were defined within the context of a system. Figure 1 shows one example of how the food safety management system is connected. The standard provides numerous examples of how the elements link together to form the food safety management system. The major elements of ISO 22000 are described in Supplementary Table 4.
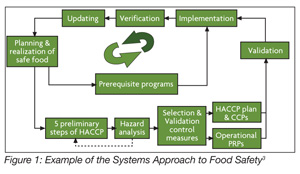
The Major Elements of ISO 22000:2005 —Food Safety Management Systems — Requirements for Any Organization in the Food Chain
| Food safety management system Documentation General requirements Documentation requirements Management responsibility Management commitment Food safety policy Food safety management system planning Responsibility and authority Food safety team leader Communication Emergency preparedness and response Management review Resource management Provision of resources Human resources Infrastructure Work environment Planning and realization of safe products General Prerequisite programs Preliminary steps to hazard analysis Hazard analysis Establishing operational prerequisite programs Establishing the HACCP plan Updating of preliminary information and documents specifying PRPs and the HACCP plan Verification planning Traceability system Control of non-conformity Validation verification and improvement of the food safety management system General Validation of control measure combinations Control of monitoring and measuring Food safety management system verification Improvement |
Source: ISO. 2005. Food safety management systems – Requirement for any organization in the food chain. ISO 22000:2005. International Organization for Standardization, Geneva, Switzerland.
CIES and GFSI Contributions to Food Safety Standards
The CIES (Comité International d’Entreprises à Succursales) or The Food Business Forum was founded in Europe in 1953 to represent food retailers. A U.S. office was opened in 1956. Over the years, the CIES continually expanded its membership to include other associations, service providers to the retailers, food manufacturing suppliers and cooperatives. The organization currently has over 400 members from 150 countries.
In 2000, the CIES launched the GSFI, which originally started with the major European food retailers. The primary decision-making body is the GSFI Foundation Board, and its original objective was to have a common set of requirements for the European food safety schemes. This has expanded to “continuous improvement in food safety management systems to ensure confidence in the delivery of safe food to consumers.”[4]
Over the years, the Board has expanded to 16 professionals who represent retailers, foodservice companies and food manufacturers around the world. Nine members are from Europe, six members are from the U.S. and one member is from Asia. Membership on the board is by invitation only.
The GFSI has issued a guidance document that lists the requirements for a “conforming food safety management standard.” It states that such a standard must include the following key elements:
• Food safety management system
• Good food practices
• HACCP, based on either the Codex definition of HACCP or the National Advisory Committee for the Microbiological Criteria for Foods definition of HACCP
The guidance document’s focus is food safety. The writers have intentionally excluded a number of issues, including product quality, environment, social/ethical issues, animal welfare, sustainability, biotechnology and innovative processes. Supplementary Table 5 at provides further elaboration on the elements of a food safety management system. The document shows how existing standard owners can seek alignment to the requirements, provides a framework for benchmarking and offers guidance on the operations of the certification process. GSFI emphasizes that the guidance document, which is updated every five years, is not a standard.
Table 5:
Key Food Safety Management Systems Elements as Required by GFSI Guidance Document Edition 5
|
KEY REQUIREMENTS Food safety policy Food safety manual Management commitment Management review including HACCP verification Resource management General documentation requirements Specifications Procedures Internal audits Corrective action Control of non-conformity Product release Purchasing Supplier performance monitoring Traceability Compliant handing Serious incident management Control of measuring and monitoring devices Product analysis GMP REQUIREMENTS Facility environment Local environment Facility layout and product flow Fabrication or construction of facility Equipment Maintenance Staff faculties Foreign body and chemical contamination risk Housekeeping cleaning and hygiene Water quality management Waste management Pest control Personal hygiene Training Protective clothing Cross-contamination risk Segregation — to avoid cross-contamination Stock management or rotation Medical screening Veterinary medicine — ensure that veterinary residues do not exceed published maximum residue levels (MRLs) Pesticide, herbicide and fungal control — pesticide residues do not exceed published MRLs Post-harvest treatment — NA Feedstuffs - NA AUDITOR REQUIREMENTS Auditor competencies Education Work experience — min 2 years in food area Formal auditor training and experience Initial training Continual training Auditor attributes Source: www.ciesnet.com GFSI uses two ISO standards to manage the certification schemes. The first is ISO Guide 65, and the second is ISO 22003.4 The certification scheme consists of the following components: • The food safety management system standard • A clearly defined scope • A certification system that consists of requirements for auditors, a statement of the approximate duration and frequency of the audits, and the minimum content of the audit report At present, four standards have met all of the benchmarking requirements for GFSI. These standards are BRC issue 5; IFS version 5; Dutch HACCP option B; SQF 1000 Edition 4 (GFSI recognition at Level 3) and 2000 Edition 6 (GFSI recognition at Level 2). ISO 22000 and GFSI The GFSI Technical Committee has looked at ISO 22000. It has identified the following three areas where differences exist between ISO 22000 and the GFSI Guidance Document.[4] Those differences are the following: • Lack of defined prerequisite programs in ISO 22000 • Differences in accreditation between ISO 22000 and GFSI; ISO 22000 accreditation scheme is to ISO 22003 and ISO 17021 while GFSI is to ISO 22003 and ISO Guide 65 • Ownership and accountability issues At the February 2009 meeting of the GSFI in Barcelona, Dr. Cor Groenveld, Chairman of the Board Foundation for SCV in the Netherlands, presented a certification scheme that is designed to harmonize the differences between ISO 22000 and GFSI benchmark requirements.[5] The critical parts of the SCV proposal include the following: • A certification scheme using ISO 22000, PAS 220 (see sidebar What is PAS 220 ), ISO 22003 and ISO Guide 65 as the standards for certification. • A focus on the scope of certification on food safety audits, ensuring transparency of the certification process and operating under a not-for-profit approach. Dr. Groenveld also discussed how ISO 22000 and other prerequisite program standards could be used to provide certification schemes for other parts of the food chain, including packaging, manufacturing, animal production and plant production. The GFSI Technical Committee is currently determining if this audit scheme meets the GFSI benchmarking requirements for a food safety management system. Editor’s note: It was expected that the Technical Committee would announce its decision in May, but at press time the decision had not yet been made public. Comparison of the Standards Whenever the GFSI standards are presented at various international forums, there is a discussion on which standard is best. Various criteria are used in the comparison. One comparison was made by Dr. Mike Robach, vice president of corporate food safety and regulatory affairs at Cargill.[6] Comprising the basis of the comparison were 67 key criteria for food safety, regulatory and quality management that are critical to Cargill. Dr. Robach reported the following scores at a USDA-FSIS meeting: • ISO 22000:2005 96% • BRC version 4 93% • Dutch HACCP option B 81% • IFS version 4 94% • SQF 2000 level 3 edition 5 91% • GFSI guidance document version 5 91% Another comparison among the various standards was made by Kathy Wybourn, director of food safety solutions, and Andrea Niemann-Haberhausen, manager of food services, both at DNV. They compiled a matrix, illuminating what is involved in each standard (BRC, IFS, and SQF): what kind of prerequisite preparations are needed, auditor qualifications, how the audit grading systems work, time frames for conducting audits, and how much each type of audit would cost.[7] Thus, one can ask the question, are there any real differences between the standards? All of the standards are relatively equivalent but each tends to have its own emphasis. ISO 22000 takes a strong management system approach. These are requirements that link the food safety management system to other business processes. It also emphasizes linking the elements of the standard to form a management system. The standard has strong internal and external communication requirements and a number of feedback loops to improve and update the food safety management system. BRC, IFS and SQF 2000 have more specific or prescriptive requirements for Good Manufacturing Practices (GMPs), or prerequisite programs that make them stronger with regard to operational issues such as post-process contamination control. This is not insignificant. The BRC has 326 requirements in its standard, the IFS has 250 requirements in its standard and SQF 2000 has a checklist with 41[8] single checkpoints for document review and 429 single checkpoints for the on-site review.[7] The proper design, implementation and maintenance of the prerequisite programs also is significant. When FDA recently evaluated the root cause of Class I and Class II recalls,8 the agency reported that 88% of the recalls were linked to failures in GMP programs (Figure 2). If one looks at the most recent, high-profile recalls that have occurred in the U.S., the root causes of these recalls appear to be failures of GMPs rather than failures in the HACCP system. ISO 22000, SQF 2000, BRC and IFS require the development of strong GMP programs that are validated, verified and improved. 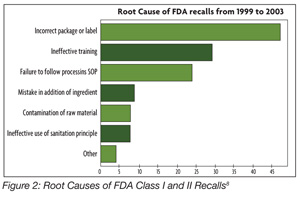
Credit: John G. Surak and Kathy L. Gombas
Number differences can lead to confusion between the various standards. When comparisons are made, it is important to compare not only the standards but also the guidance documents. The guidance document for ISO 22000 is ISO 22004. In addition, both SQF 2000 and BRC have guidance documents. These documents present information on how the standard should be interpreted. Some individuals tend to misread these documents. The normative standard is written using the words “shall” or “must.” The guidance documents are written using the word “should.” These guidance documents present a state-of-the-art interpretation. Many auditors and certification bodies expect that the food processor will implement the interpretation in the guidance. There is an exception if the food processor implements a component of the food safety system that exceeds the description in the guidance documents and is demonstrated with a validation document.
ISO 22003 • Audit experience—the auditor must have a minimum of 12 days auditing food safety management systems |
What is PAS 220?
PAS stands for Publicly Available Specification. A PAS is a document based on the British Standard model and used to standardize best practices on a specific subject for the benefit of an industry.
National and international standards must reach full consensus between all of the stakeholders of the standard. The PAS invites comments from stakeholders, but may not incorporate the comments. As a result, a usable document can be developed in a short period of time (about eight months), thus allowing for implementation by stakeholders. In contrast, it typically takes five years to complete the initial development of an international standard.
PASs can be elevated to national or international status by submitting them through the standards development process. Once a national or international standard is issued, British Standards will withdraw the PAS.[1]
PAS 220:2008[2] was developed to be used with ISO 22000 to provide further detail of prerequisite programs as found in element 7.2.3. This specification was developed by food safety professionals representing the Food and Drink Foundation, McDonald’s, General Mills – Europe, Lloyd’s Register Quality Assurance, the French National Association of Food Industries and ProCert Organisme Certifacteur. The standard also had support from Dannon, Kraft Foods, Nestlé and Unilever.
The standard addresses the following prerequisite programs:
• Construction and layout of buildings
• Layout of premises and workspace
• Utilities – air, water, energy
• Waste disposal
• Equipment suitability, cleaning and maintenance
• Management of purchased materials
• Measures for prevention of cross-contamination
• Cleaning and sanitizing
• Pest control
• Personal hygiene and employee facilities
• Product recall procedures
• Warehousing
• Product information and consumer awareness
• Food defense
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →






