Delivering Quality Nutritional Products from Supply to Shelf
Companies delivering high-quality nutritional beverage products to consumers face many obstacles. Early in the product development stage, challenges arise as companies evaluate new ingredients and initiate working relations with new suppliers. Food companies must determine whether these ingredients—and their suppliers—can reliably meet increasingly demanding safety requirements. Companies and their suppliers also encounter varied global standards and unique testing requirements for each country or region. Lastly, companies must ensure they are properly testing products to comply with various contaminant requirements using appropriate testing methods that ensure safe products around the world. Abbott has managed these challenges for many years and continues to be a premier manufacturer recognized for its high-quality beverage products, including its Ensure and Glucerna shakes.
Supply chain challenges are always evolving as companies expand globally. Companies are faced with changing supplier dynamics and workforce demands, while also navigating differing regulatory requirements. As the global food supply chain expands, with new manufacturing plants and global distribution of products, companies face opportunities—and challenges—that didn’t exist before. In addition, the regulatory environment is evolving rapidly, particularly in developing markets as governments implement new or updated food safety regulations.
Abbott has successfully entered several new markets and is expanding its presence in key emerging markets, including Vietnam, India and China, by investing in these markets and working with employees and suppliers to improve food safety standards and access to technology. Abbott believes food safety should be defined the same way around the world, but that is not reality. How a safe practice is defined in one region may not align with the definition in other regions. Each country or region can have differing food import regulations or testing requirements that usually call for very specific technologies. Establishing consistent and common global regulations and standards can help break down many of the barriers to expanding and strengthening the global food supply chain.
Food safety starts with the manufacturer and is an important element of brand management. Food and beverage manufacturers who wish to expand their business should start by building brands that are known for their commitment to high quality and safety. Achieving this level of quality requires food safety standards that are clear, simply stated and internationally accepted. Unified standards offer predictability and consistency for manufacturers, which allows for greater efficiency and continued food safety effectiveness.
Industry Collaboration
In the past 10 years, we’ve seen a greater focus on industry collaboration to set global safety standards. One example in which Abbott has played a role is the SPIFAN (Stakeholder Panel on Infant Formula and Adult Nutritionals) initiative to help develop global reference analytical methods for infant and adult nutritional products. The use of harmonized analytical test methods, for nutrients as well as contaminants, is a part of the overall food safety program.
Formed in 2010, SPIFAN is a project governed by the American Association of Analytical Chemists (AOAC) and supported by the infant formula industry to establish international reference materials for key nutrients found in infant and adult nutritionals. These globally accepted standards can help facilitate international trade of nutritional products for the benefit of all stakeholders.
When food manufacturers collaborate to develop standardized processes and practices, it can have many positive effects:
• Reduces regulatory food trade disputes with internationally accepted analytical methods
• Enhances harmonization and avoids duplication of work
• Promotes an understanding among manufacturers, authorities and contract laboratories on analytical methods
• Harmonizes compliance testing of products
A company’s divisions must also work closely together, especially when the company has multiple locations. Abbott implemented a standardized laboratory training program that is the same for every employee around the world. The company seeks to harmonize training on test methods and procedures; if it is protocol for one site, it should be accepted at all sites. This allows for an efficient training rollout and consistency of activity at all of our manufacturing locations.
Working with Suppliers
The theme of harmonization and standardized education extends to our suppliers. Although suppliers may not be part of a company’s organizational structure, they should be evaluated against the same standards.
When looking to select a new supplier, companies should conduct in-depth audits of potential suppliers. A review of quality systems, improvement measures and programs, financial viability and overall sustainability should be completed to ensure the selection of high-quality suppliers.
After a supplier is selected, it should have full knowledge of a manufacturer’s food safety procedures and how its product or materials will be used in the finished goods. Companies should also take the time to work with their suppliers before concerns arise. This collaborative approach will benefit all stakeholders and can create a stronger supplier relationship.
Quality Testing of Ingredients
The product formulation of nutritional drinks is complex and often uses ingredients sourced from different suppliers. As a result, companies must be diligent in their quality testing to ensure safe and high-quality finished products. Given the stringent regulations that govern the sale of nutritional products, food safety is a critical part of a company’s business.
An effective food safety program for nutritional products begins with minimizing the risk of contaminants entering into products. Screening during the qualification stage is technically complex. For some categories of foods, the safety regulations for finished products are better defined than the regulations for the ingredients in them. This can cause confusion concerning the requirements for ingredient testing. A strong understanding of the formulation, production and regulatory requirements for the finished nutritional product is required to ensure appropriate testing during ingredient qualification.
Companies should consider the following elements as part of an effective food safety program:
• Supplier qualification and approval
• Ingredients testing for both purity and safety
• Ingredients surveillance testing
• Finished product testing
Keeping Finished Beverage Products Safe
Once a supplier is selected and ingredients are sourced, a company or its suppliers may test the ingredients for compositional purity (protein or carbohydrate profile, fatty acid composition) and confirm the absence of chemical contaminants.
Testing for chemical contaminants in ingredients requires collaboration between an organization’s quality, regulatory, analytical, medical safety and manufacturing groups. The main ingredients used in nutritional beverages are of dairy, plant, marine and mineral origin, each of which can introduce a diverse set of chemical contaminants. The list of ingredients vs. potential contaminants is listed in Table 1.
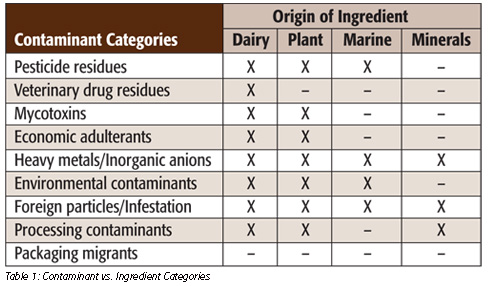
The contaminants in Table 1 include compounds that are approved for use—pesticides and veterinary drugs—and compounds that are banned for use in some countries, including growth promoters such as steroids.
Because of the complexity of tracking chemical contaminants in ingredients and products, and the incomplete regulatory definition of acceptable levels of contaminants, testing ingredients is challenging.
Identifying Issues with a Cross-Functional Team
Ensuring safe products impacts multiple areas of the business; therefore, an effective food safety program must include cross-functional teams with members from quality assurance, regulatory affairs, analytical and medical safety groups. See Figure 1 for an example of a strong cross-functional team structure.

Three major cross-functional teams must work together:
Quality assurance team: Develops sustainable quality systems, programs, quality reviews and ensures safe ingredient sourcing.
Regulatory affairs team: Provides clarity on the list of regulated analytes, or substances, for ingredients in different markets. When no clear regulatory guidance is available, this team conducts a risk-based assessment and provides target lists for ingredients along with relevant detection thresholds.
Analytical team: Develops methods required for detecting the contaminants that may be present in ingredients. The analytical methodology involves broad screening techniques with the required degree of sensitivity of detection, as well as confirmatory methods for the regulated analytes.
While the cross-functional approach necessary to ensure safe food is well known among food safety professionals, broader audiences often do not recognize the role that regulatory affairs and analytical teams play in ensuring safe food and the challenges they face.
Regulatory Affairs
Managing chemical contaminants in the supply chain process includes producing beverages that are safe and meet all global regulatory requirements. This is a complex challenge for the regulatory affairs team, which requires control over ingredients from a wide variety of food categories.
Conducting quality tests not only in the finished product, but also in the ingredients going into the finished products, is important for the following reasons:
To meet finished product contaminant standards, it is easier and more cost effective for companies to test individual ingredients before developing a product that does not meet product regulations. Potential issues can be addressed at an ingredient level, versus investigating potential issues in the completed product formulation.
Regulatory agencies are increasingly requiring companies to show the regulatory compliance of ingredients they use, in addition to the products they are manufacturing. For example, the European Union has indicated (in EC 1881/2006) that “foodstuffs not complying with the maximum levels set out in the Annex shall not be used as food ingredients.”
This has been used as the basis for enforcement; thus, companies must ensure that their products—and ingredients—meet the contaminant specifications in this regulation. The emphasis on regulating chemical contaminants in ingredients as well as products is gaining more traction globally.
Challenge: No Global Standards
As we mentioned before, a challenge with overseeing chemical contaminant regulations is that they do not exist for every ingredient. Due to differences in priorities, countries often focus their regulations on different chemical contaminants or the same chemical contaminants but in different food categories. For example, the control of mycotoxins in a hot and humid tropical country may be a higher priority than in a country from a northern latitude.
To address this, global food companies must establish a strategy for coping with these inconsistencies. One strategy is to adopt very restrictive requirements. This helps ensure that all products and ingredients meet global regulations. However, this approach can unnecessarily limit a company’s supply options in order to meet regulations that are not relevant, either to the category limits of some final products or to the regulatory limits of the countries where the products are marketed.
Another option is to create a system that allows for differences between products in each country or region. However, this requires a possibly elaborate system and significant resources to manage the continually changing global regulatory landscape. A better solution may be to create a hybrid system by grouping product categories or global regulations.
While regulations often define the contaminant level requirements and which contaminants belong in which food categories, in most cases the regulations provide little guidance on the frequency of testing needed. This gives nutrition companies the ability to develop risk-based systems for testing that prioritize high-risk contaminants. Such flexibility allows a company to employ a conservative approach but requires fewer resources because the company is not faced with attempting to comply with varying requirements.
Solution: Risk-Based Target Lists
Abbott believes a robust, risk-based quality testing program will help companies ensure that products and ingredients meet global regulatory limits and that their products are compliant.
Creating categories of food ingredients (e.g., proteins, fats and oils, carbohydrates) will help companies organize the ingredients they use. Categorization aids in development of a generalized risk assessment strategy, simplifying the processes while still providing a conservative approach for ensuring ingredient and product compliance with regulations.
For example, while soy oil, corn oil, canola oil, sesame oil and sunflower oil may have some differences in the contaminants possibly present, because they are similar agricultural commodities that undergo similar processing, a single target list could be developed. However, not all commodities are created equal, and therefore a company will want to focus resources on the highest-risk commodities in each ingredient category.
By establishing a scientific, risk-based approach, companies can reduce the risk of ingredients and products not meeting global regulations, demonstrate to regulators that the risks of contaminants are being addressed and provide confidence to the public that the food supply is safe.
Defining Ingredient Risk
Many factors can be considered when evaluating how often an ingredient category should be tested, including:
• How prevalent is an ingredient category in a typical product or product category? For example, if oils make up more than 80 percent of a typical product and carbohydrates make up less than 1 percent of a typical product, then a company may choose to test its oil ingredients more frequently.
• Does the ingredient category historically have issues with contaminants? Some contaminants are present only in raw agricultural commodities, such as milled grain, but many are eliminated when they are processed into ingredients such as glucose or maltodextrin. Therefore, a company may test the raw agricultural commodities more frequently than processed carbohydrates.
The risk assessment for individual ingredients within each category can be further refined using information about the source of the ingredient. Criteria to consider include food safety practices and regulations in the ingredient’s source country and a history of contamination or adulteration in that country or region.
Using the aforementioned criteria for ingredient category risk, in conjunction with specific ingredient risk, a company can establish a scientifically rationalized, risk-based process for identifying the ingredients that pose the highest risk and then focus the most resources on testing those ingredients.
Analytical Testing
Abbott’s analytical team uses a variety of technology platforms to assess organic and inorganic compounds and contaminants in ingredients.
Oftentimes, a company must combine its analytical platforms to test compounds in the broadest possible range. Because of the complexity of ingredients and diversity of contaminants, it is desirable to have a universally applicable sample extraction procedure that can obtain a range of compounds. The commonly used sample preparation technique for the extraction of small molecules, including many chemical contaminants, is the QuEChERS (quick, easy, cheap, effective, rugged and safe) procedure.[1]
Other techniques are used to test for known contaminants, including:
• Volatile and semivolatile substances, such as organic solvents and pesticides
• Foodborne trace chemical contaminants, including pesticides, mycotoxins, economic adulterants, veterinary drug residues, packaging chemicals and other related compounds[2, 3]
• “Unknown” compounds present in food ingredients[4]
• Heavy metals and inorganic anions
Analytical testing continues throughout the supply chain. The qualified ingredients are then used for manufacturing commercial products and in plants operating under Good Manufacturing Practices manufacturing controls, which ensure safety and quality of the finished product. These ingredients are periodically analyzed as part of a surveillance program to ensure consistent quality and safety.
The finished beverages are similarly tested for process residuals, potential packaging migrants and other select
contaminants.
A Comprehensive Approach to Testing
The use of sophisticated analytical technologies to screen ingredients for contaminants offers the best possible strategy for ensuring the safety of nutritional products, although it is not entirely without issues.
For instance, given the sophistication and sensitivity of some analytical technologies, it’s possible to detect extremely low levels of extraneous chemicals in ingredients and products—well below any recognized contamination threshold. Additionally, the chemical complexity of ingredients and products can produce false-positive detections of a contaminant, which may not be relevant to the actual safety of the ingredient or product.
Therefore, food safety programs must not rely solely on technologies but should combine sound analytical methodology with regulatory framework requirements and medical safety evaluations.
Ensuring Safety from the Start
Ensuring the success of nutritional beverage products manufactured, distributed and sold all around the globe demands the discipline of many functional areas working together and providing timely input from their own area of expertise.
The process begins early in the product development stage and extends beyond a company’s walls. It requires collaboration across many functions and a large investment to set up the tools and processes. Abbott encourages industry to work with government agencies to address some of the challenges—such as differences between regulations—to help more companies deliver on the promise of high-quality and nutritious products.
Bradd Eldridge, B.A., M.B.A., is the director of quality with Abbott Nutrition.
Murali Reddy, M.Sc., currently heads the food safety and analytical research group at Abbott Nutrition.
Paul Hanlon, Ph.D., DABT, is an associate director of regulatory affairs at Abbott Nutrition.
References
1. Anastassiades, M., S.J. Lehotay, D. Stajnbaher and F.J. Schenck. 2003. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431.
2. Turnipseed, S.B., J.M. Storey, S.B. Clark and K.E. Miller. 2011. Analysis of veterinary drugs and metabolites in milk using quadrupole time-of-flight liquid chromatography-mass spectrometry. J Agric Food Chem 59(14):7569–7581.
3. Gallart-Ayala, H. and O. Núñez. 2013. Recent advances in LC-MS analysis of food-packaging contaminants. TrAC Trends in Analytical Chemistry 42:99–124.
4. Díaz, R., M. Ibáñez, J.V. Sanchoa and F. Hernández. 2012. Target and non-target screening strategies for organic contaminants, residues and illicit substances in food, environmental and human biological samples by UHPLC-QTOF-MS. Anal Methods 4:196–209.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →






.webp?t=1721343192)