2020 STATE OF THE INDUSTRY
State of the Industry in Food Safety: Facility Management & Design in the COVID-19 Era
Federal regulators and industry associations issue a wave of new guidance to navigate the ongoing COVID-19 pandemic.
On December 31, 2019, the Wuhan Municipal Health Commission, Wuhan City, China, reported several pneumonia cases with unknown causes, eventually identifying a novel coronavirus called SARS-CoV-2. Before the conclusion of January, this coronavirus had breached U.S. borders. Since that time, the COVID-19 pandemic has wreaked havoc on the state of public health.
According to the “COVID Data Tracker” from the Centers for Disease Control and Prevention (CDC), as of September 10, 2020, the COVID-19 pandemic in the U.S. has resulted in:
- 6,343,4562 total cases
- 190,262 total deaths
According to the “Coronavirus Disease (COVID-19) Dashboard” from the World Health Organization (WHO), as of September 10, 2020, the COVID-19 pandemic globally has resulted in:
- 27,738,179 total cases
- 899,916 total deaths
The global and domestic economy has also seen significant impact from the pandemic. According to the U.S. Bureau of Labor Statistics, the domestic unemployment rate skyrocketed from 3.5 in February 2020 to 14.7 in April 2020. During those three months, the consumer price index dropped from 0.1 to -0.8. According to the Bureau of Economic Analysis, part of the U.S. Department of Commerce, real gross domestic product decreased at an annual rate of 31.7 percent in the second quarter of 2020 spurred by the widespread “stay at home” orders issued during March and April (“Gross domestic product, 2nd quarter 2020 (second estimate); corporate profits, 2nd quarter 2020 (preliminary estimate).” The Bureau of Labor Statistics also reports profits from domestic production decreased $276.2 billion in the first quarter of 2020 and $226.9 billion in the second quarter.
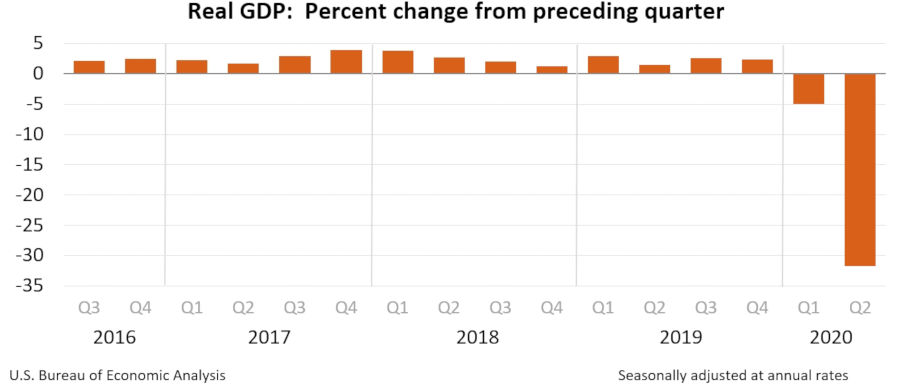
Courtesy of U.S. Bureau of Economic Analysis | View full-size chart
Despite the pronounced economic downturn in the ongoing wake of the pandemic, food producers—as essential businesses—continued operation where possible, but have faced significant challenges.
Disruption of the century
“COVID-19 is the single largest public health impacting event in a century,” says Steven Mandernach, executive director, Association of Food and Drug Officials, York, PA, and one of the presenters of “COVID-19: The New Normal for the Food Industry,” the opening general session of the 2020 Food Safety Summit. “This event has disrupted many of the ways Americans interact everyday with food including dining, grocery shopping, and travel. Further, we are likely to continue dealing with COVID-19 into 2021.”
The public health and regulatory communities were not prepared for a pandemic of this magnitude, says Mandernach. “Further, the public health and regulatory communities would not have anticipated the level politics and ideology would play in all levels federal, state, and local COVID-19 responses. Science and public health often were not the prevailing criteria used by federal, state, and local policy makers to make decisions.” He notes the lack of a uniform approach to this national challenge led to a haphazard response, eroding public confidence and reducing the effectiveness of limiting the spread.
“Regulators and public health officials simply could not respond quickly enough to provide the needed information to the food industry for a variety of reasons: lack of clear data, cumbersome clearance processes, and prohibitions by political officials,” says Mandernach.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
Mitigating risk
As essential businesses like food production returned to work after initial shutdowns in early 2020, many faced public health issues related to person-to-person viral transmission. “Industries across the U.S., including food, forgot the inherent risk of having many people close together in a pandemic with a virus that transmits so effectively in close quarters, creating significant challenges.”
FDA and the Occupational Safety and Health Administration (OSHA) provided an “Employee Health and Food Safety Checklist for Human and Animal Food Operations during the COVID-19 Pandemic.” FDA and OSHA developed the checklist for FDA-regulated human and animal food operations to assess operations during the COVID-19 pandemic—and particularly when restarting operations after a shutdown. Regulators developed the document to provide guidance for facilities growing, harvesting, packing, manufacturing, processing, or holding human or animal food regulated by FDA, including produce, seafood, milk, eggs, grains, game meat, and other raw materials or ingredients, as well as their resulting human or animal food products.
The guidance document notes: “Currently there is no evidence of food or food packaging being associated with transmission of COVID-19. However, according to CDC and OSHA, the work environments—processing lines and other areas in busy plants where workers have close contact with coworkers and supervisors—may contribute substantially to potential worker exposures.”
This vital document from FDA and OSHA includes a wealth of links to supporting industry guidance, as well as the following checklists employers should consider when assessing operations during the COVID-19 pandemic:
- General practices
- Facility safety
- Personnel health and safety, including use of personal protective equipment (PPE)
- Work environment configuration
The document also includes a checklist for addressing personnel who are symptomatic or develop symptoms at work, as well as detailed steps to follow for employee COVID-19 exposure, including investigation and testing protocols.
The guidance addresses the need to analyze any disruption of existing food safety or HACCP plans caused by personnel or facility changes stemming from COVID-19 mitigation protocols. This can include changes to personnel requirements, changes to the supply chain, introduction of new hazards, and changes to operations or procedures.
Effective and validated facility disinfection is an instrumental component of COVID-19 pandemic mitigation. The Environmental Protection Agency (EPA) maintains a list of disinfectants approved for effective use against the COVID-19 virus (see “Disinfectants for Use against SARS-CoV-2 (COVID-19)”). EPA also offers guidance for disinfection in “Guidance for Cleaning and Disinfecting Public Spaces, Workplaces, Businesses, Schools, and Homes.”
Foodservice has seen significant disruption due to the established guidance for social distancing. Restaurants have also shifted operations to include a pronounced strategy focused on carryout and delivery, and sometimes retail sales.
The National Restaurant Association has published a guidance document outlining recommended procedures for reopening restaurants during the COVID-19 pandemic (see “COVID-19 Reopening Guidance: A Guide for the Restaurant Industry”). The guidance document includes detailed sections on topics like cleaning and sanitizing, monitoring employee health and personal hygiene, and social distancing.
Summary of Best Practices for Retail Food Stores, Restaurants, and Food Pick-Up/Delivery Services During the COVID-19 Pandemic
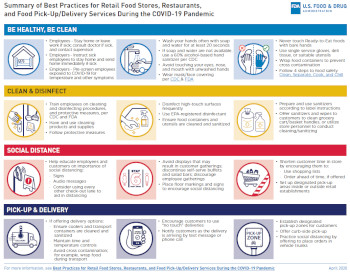
Courtesy of FDA
FDA has provided a re-opening checklist for retail food establishments, addressing key food and public safety practices (see “Best Practices for Re-Opening Retail Food Establishments during the COVID-19 Pandemic”). The checklist covers:
- Facility operations
- Water, plumbing, and ice
- Disinfecting food-contact and non-food-contact surfaces
- Handwashing stations
- Employee health and screening
- Social distancing
In “Best Practices for Retail Food Stores, Restaurants, and Food Pick-Up/Delivery Services During the COVID-19 Pandemic,” FDA goes into additional detail, sharing information about best practices to operate retail food stores, restaurants, and associated pick-up and delivery services during the COVID-19 pandemic to safeguard workers and consumers. The guidance addresses key considerations for safe handling and/or delivery of foods to the public, as well as best practices for employee health, cleaning and sanitizing, and PPE.
In the guidance for managing foodservice or retail food operations, FDA notes the following:
- Wash, rinse, and sanitize food contact surfaces, dishware, utensils, food preparation surfaces, and beverage equipment after use
- Frequently disinfect surfaces repeatedly touched by employees or customers such as doorknobs, equipment handles, checkout counters, and grocery cart handles
- Frequently clean and disinfect floors, counters, and other facility access areas using EPA-registered disinfectants
- Prepare and use sanitizers according to label instructions
The FDA document also includes detailed guidance related to helping customers maintain good infection control and social distancing, as well as safely managing food pick-up and delivery.
Audits and inspections
The rise of the COVID-19 pandemic has disrupted standard protocols for facility audits and inspections, with remote and hybrid options arising to replace or supplement in-person inspections where possible.
BRCGS. Full onsite audit options for announced and unannounced audit programs remain available where possible. BRCGS has also developed alternatives:
Food Safety Summit on Demand

COVID-19: The New Normal for the Food Industry
Two-hour session, one hour of moderated breakout groups
Presenters:
- Steve Mandernach, Association of Food and Drug Officials
- Jorge Hernandez, The Wendy’s Co.
- Craig Wilson, Costco
- Joan Menke-Schaenzer, Van Drunen Farms/FutureCeuticals
- Glenn Stolowski, HEB
- Lee-Ann Jaykus, Ph.D., North Carolina State University
• Blended audits are now a permanent option, executed in two parts—a remote online assessment of some or all of the documentation followed by a shorter on site audit, thereby reducing the time an auditor spends onsite
• Where it is not possible to carry out an onsite audit of an existing certificated site, processors can extend the certificate for up to six months based on a risk assessment and review
• Full remote assessment (not GFSI benchmarked) is available for sites when certificate extension has expired and an onsite audit is still not possible due to COVID-19 restrictions or where a site does not need a GFSI-recognized certificate—the remote audit includes a review of internal audit results, a remote documentation revue, and a video audit of production/storage facilities
BRCGS has also published a separate COVID-19 Additional Module assessment standard to provide assurance around the management of COVID-19 risks, undertaken as a remote audit and focusing on areas of food safety plans potentially at greater risk because of changes enforced to address COVID-19, such as changes to the supply chain.
SQF. SQFI has waived the unannounced audit policy all audits scheduled on or before December 31, 2020. SQFI has also implemented processes for risk assessments, and can defer certification due to extenuating circumstances, allowing for a one-time six-month extension, aligning with GFSI. Certifying bodies must conduct a risk assessment to determine the necessity of a certificate extension. SQF policy requires the certifying body to assess the risk for continuing certification during an extraordinary event and request information from sites, as necessary. Risk assessment might dictate a need for remote activities to maintain the site’s certification (remote activities do not replacement the need for a recertification audit).
FDA. In March 2020, FDA temporarily postponed all domestic and foreign routine surveillance facility inspections. FDA will request existing inspection reports from trusted foreign regulatory partners through mutual recognition and confidentiality agreements, as well as requesting records and other information from applicants, facilities, and other inspected entities.
In July 2020, FDA began to work toward resuming prioritized domestic inspections using its COVID-19 Advisory Rating system to determine what categories of regulatory activity can take place in a given geographic region. FDA is continuing, on a case-by-case basis, to conduct “mission-critical” inspections where possible to do so safely, resuming prioritized domestic inspections, which generally include pre-approval and surveillance inspections. FDA will pre-announce any prioritized domestic inspections to help ensure the safety of the investigator and the inspected business’s employees, providing the safest possible environment to accomplish FDA’s regulatory activities, while also ensuring the appropriate staff are on-site to assist FDA staff with inspection activities.
Foreign pre-approval and for-cause inspection assignments not deemed mission-critical remain temporarily postponed, while those deemed mission-critical will potentially face inspection on a case-by-case basis.
FSIS. Meat, poultry, and processed egg facility inspections have continued through the COVID-19 pandemic. All FSIS-regulated establishments must have sanitation standard operating procedures (SSOPs) to prevent direct contamination or adulteration of product. Those SSOPs will help prevent the spread of respiratory illnesses like COVID-19.
In the event of a diagnosed COVID-19 illness, FSIS is encouraging establishments to follow the recommendations of local public health authorities regarding notification of potential contacts. FSIS will keep its lines of communication open to address evolving situations.
FSIS has delayed both U.S. and foreign country audits in accordance with State Department guidance. FSIS continues to monitor the situation and will evaluate the feasibility of its upcoming audits as the situation evolves, including reviewing State Department guidance on foreign travel.
COVID-19 Industry Resources
Grower/Farmer
• “Interim Guidance from CDC and the U.S. Department of Labor for Agriculture Workers and Employers”
Manufacturer/Processor
• FDA and OSHA, “Employee Health and Food Safety Checklist for Human and Animal Food Operations during the COVID-19 Pandemic”
• Interim Guidance from CDC and OSHA, “Meat and Poultry Processing Workers and Employers”
• Interim Guidance from CDC and OSHA, “Protecting Seafood Processing Workers from COVID-19,” developed in consultation with FDA
Distributor
• FDA, “Best Practices for Retail Food Stores, Restaurants, and Food Pick-Up/Delivery Services during the COVID-19 Pandemic”
• DOT, “Coronavirus Resources at the Department of Transportation”
Retailer/Foodservice
• FDA Safety Checklist, “Best Practices for Re-Opening Retail Food Establishments during the COVID-19 Pandemic”
• National Restaurant Association, “COVID-19 Reopening Guidance: A Guide for the Restaurant Industry”
• FDA, “Best Practices for Retail Food Stores, Restaurants, and Food Pick-Up/Delivery Services during the COVID-19 Pandemic”









