Food Safety
How to manage food labels to ensure allergens are clearly noted on any product
Use of the wrong package or label is one of the most common causes of allergen recalls

“May contain” statements shouldn’t be used in place of strong sanitation and GMP programs.
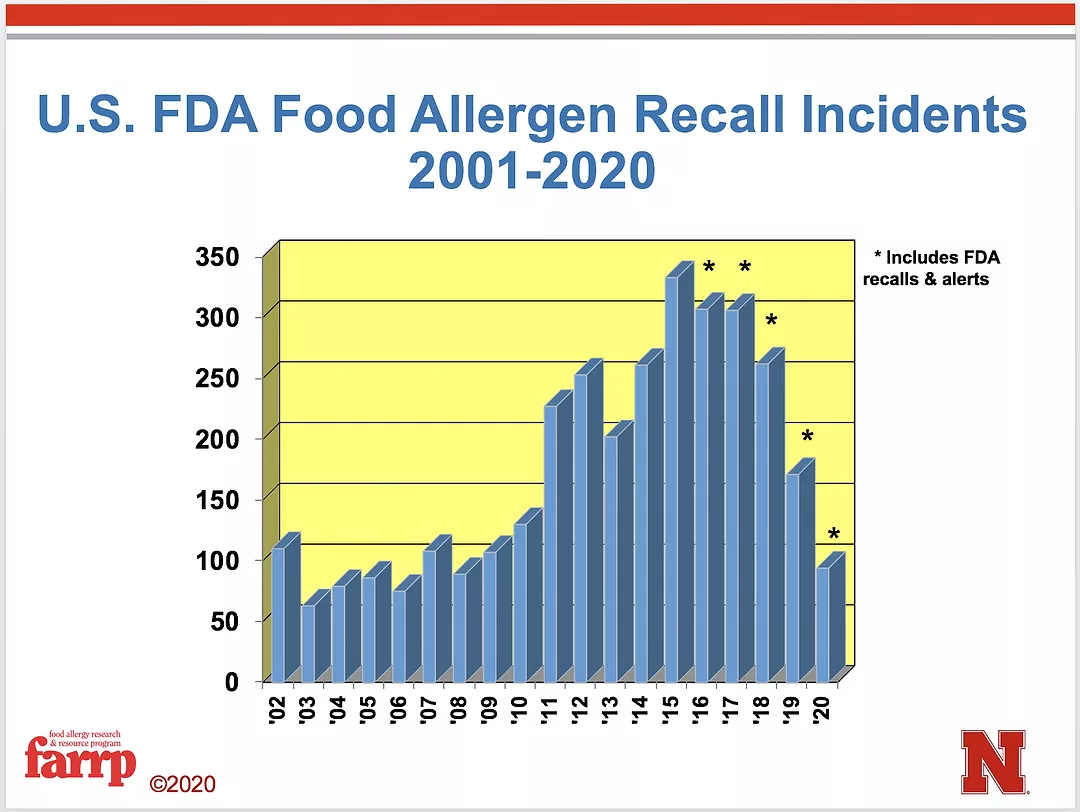
FDA recalls by year
Courtesy of the Food Allergy Research & Resource Program, University of Nebraska-Lincoln
One of the mandates that came with the enactment of the Food Allergen Labeling and Consumer Protection Act (FALCPA) in 2004 is the requirement to clearly label food allergens that are found in any food product.
The regulation defines eight allergens that must be clearly labeled, known as the Big Eight:
- Soy
- Wheat
- Egg
- Crustacean Shellfish
- Fin Fish
- Milk
- Tree Nuts
- Peanuts or Ground Nuts
These allergens comprise 90% of all food allergens, but scientists have identified over 160 compounds that will cause allergic reactions. As an example, the European Union as defined 14 allergens of concern. These include the Big Eight plus lupin, molluscan shellfish, sulfites, celery, mustard and sesame. It is imperative that allergens in foods be properly labeled as there is no cure for persons who are allergic to foods. For these sensitive persons, the only way to ensure safety is for the individual to avoid the allergen or allergens in question. If they are in a commercially prepared or processed food, they must be labeled so sensitive persons know what they can or cannot eat.
Governments the world over have enacted laws and regulations mandating allergen labeling and allergen management for the food processing industries within and shipping to their countries. This is exactly what has happened in the United States. The Current Good Manufacturing Practice Hazard Analysis and Risk Based Preventive Controls for Human Food found in 21 CFR Part 117 defines allergens as a hazard that must be managed using preventive controls.
Preventive controls are defined as those risk-based, reasonably appropriate procedures, practices and processes that a person knowledgeable about the safe manufacturing, processing, packing or holding of food would employ to significantly minimize or prevent the hazards identified under the hazard analysis that are consistent with the current scientific understanding of safe food manufacturing, processing, packing or holding at the time of the analysis.
21 CFR Part 117.135 (c) 2 defines how allergens must be managed:
(2) Food allergen controls. Food allergen controls include procedures, practices, and processes to control food allergens. Food allergen controls must include those procedures, practices and processes employed for:
(i) Ensuring protection of food from allergen cross-contact, including during storage, handling and use; and
(ii) Labeling the finished food, including ensuring that the finished food is not misbranded under section 403(w) of the Federal Food, Drug and Cosmetic Act.
So, processors have two tasks: 1) keeping unlabeled allergens out of the food or prevention of cross-contact and 2) properly labeling foods that contain allergens.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
Despite FALCPA and the preventive controls regulation noted above, allergen recalls have been the primary cause of food recalls in foods regulated by the FDA. The accompanying chart shows how allergen recalls increased since FALCPA was enacted in 2004. It is only in the last few years that the upward trend of the curve has begun to reverse itself.
The most common cause is “wrong package/label.” The processor simply used the wrong package or failed to update packaging when a formulation change was made. This is a greater problem with small processors that often do not want to throw away old packaging due to cost issues. To address potential labeling concerns, it is absolutely imperative that food processors develop, document, implement and maintain a program to properly develop, receive, store and manage labels.
Label development and printing
Labels must comply with food labeling regulations found in 21 CFR Part 101 and the Food Allergen Labeling and Consumer Protection Act. Many processors utilize a third party such as a lawyer or labeling expert to assist in the development of labels and to ensure that the labels are fully compliant with the laws and regulations.
FALCPA mandates that any allergens in the product must be clearly identified on the label. This is accomplished in one of two ways. The allergen will either be defined in the ingredients statement or in a statement at end of the statement that specifically defines the allergens in the product, e.g., contains wheat. Processors may decide to include a “may contain” statement on their label. There is nothing in the FALCPA that defines this, but many operators elect to do so.
The FDA does not support such statements, but they are not against the law. The concern is that some processors may adopt a “may contain” statement in lieu of establishing good sanitation and GMP programs. There are many different such statements that are used.
Once the first draft label has been finalized, that label should be printed and reviewed by the quality group for final approval. Once approved, it can be sent to the printer. Processors must retain a copy of the approved label for their files. These draft labels should be maintained in a secure place with the quality group. The company should also make a color copy of each label master on transparent stock.
Receipt and verification
Each and every time a processor receives a new shipment of labels, it is imperative that the company verify that the labels have been properly printed. This applies to roll stock and individually printed labels. The quality group must deveop a sampling plan to examine each lot of labels for accuracy. The transparency of the master label can be used to confirm this. It is much easier to overlay the transparent master on the new labels than to try and read them.
If a new lot is found to be out of specification, the lot must be rejected. The two options are to return the lot to the printer or destroy it. Destroying the lot is best. Why? If they are returned to the printer, there is a chance that they might end up being shipped back. Inform the supplier that the labels were defective, share a few of the suspect labels, destroy the rest and be sure that credit is received if necessary.
Storage and use
Once a lot of new labels has been verified as accurate, they should be placed into a secure and locked storage facility. The company should assign someone to manage the label storage facility. This individual should be provided with daily production schedules and issue labels to representatives of the production group. Specific lots should be signed for and, if any remain at the end of production, the extras must be returned to the storage area and signed back in. One of the responsibilities of the label manager is to maintain an accurate inventory of all labels that are in stock.
The label manager will also be responsible ensuring that old or out-of-date labels are destroyed to ensure that they will not be accidentally used at some time in the future. The use of old labels is one cause of product recalls, especially among small companies. These operations think they can save a little money but any potential savings will be eaten up with the costs of a recall.
Tools to ensure proper label usage
Once the labels or roll stock are collected for use in production, it is imperative that the production personnel put the correct label on the products being manufactured. This can be a challenge if labels for different products are very similar in design. There are a number of procedures that processors can utilize to ensure that the right labels are used.
One thing that is done in many processing operations is to collect labels at regular intervals during production, review that they are the proper label for the product in question and append these labels to the production records. Each time a label is collected, the operator should note the time, date and line number on the label. This will also provide a record verifying that the proper labels were used throughout the production shift.
Another tool used by more and more processors is a label scanner. This machine will be programmed with the barcode for the product being manufactured and will scan the code on each label. If the wrong label has been applied, the container in question will be rejected or the line will automatically stop so the mislabeled product can be removed from the line.
There is also double scanning technology. This is a system that is used in the canning industry. There are times when canned foods are brite-stacked, or stored in the warehouse but not labeled. There have been times when the wrong pallet of brite-stacked cans has been transported to the labeling line. The end result would be a can that was simply improperly labeled, where the label might read chili con carne while the can contained chile with beans. This system scans the label and the container code. If the two codes don’t properly match, the line will be stopped.
One of the most common causes of allergen recalls is the use of the wrong package or wrong label on a container. It is imperative that food processors make sure that the correct label is placed on each and every product. This is especially true when packaging foods containing allergens.
The Preventive Controls for Human Food Regulation mandates that processors packaging foods containing food allergens properly label these products with the allergen they contain. Proper labeling of allergens is a preventive as defined by the regulation and the only way that sensitive persons can be protected, so programs to manage labels should be a critical element of each processor’s food safety management system.









