Hitting FSMA benchmarks in warehousing and distribution networks


The Food Safety Modernization Act, (FSMA) represents the most significant overhaul of U.S. food safety regulations in over 70 years, says Purnendu Vasavada, professor emeritus, University of Wisconsin-River Falls. FSMA was enacted in 2011, and as of today, all FSMA rules have been finalized, with implementation now underway.
What is FSMA?
According to Vasavada, the “foundational” rules of FSMA include:
- Preventive Controls for Human Food: Requires that food facilities have safety plans that set forth how they will identify and minimize hazards.
- Preventive Controls for Animal Food: Establishes Current Good Manufacturing Practices and preventive controls for food for animals.
- Produce Safety: Establishes science-based standards for growing, harvesting, packing, and holding produce on domestic and foreign farms.
- Foreign Supplier Verification Program: Importers will be required to verify that food imported into the United States has been produced in a manner that provides the same level of public health protection as that required of U.S. food producers.
- Third Party Certification: Establishes a program for the accreditation of third-party auditors to conduct food safety audits and issue certifications of foreign facilities producing food for humans or animals.
- Sanitary Transportation: Requires those who transport food to use sanitary practices to ensure the safety of food.
The initial phase of FSMA implementation emphasized education, such as Preventive Control Qualified Individuals (PCQIs) and understanding FSMA requirements.
The FDA has started inspection of food processing plants to assess FSMA implementation, says Vasavada.
“Initially, the inspection focused on compliance with cGMPs. This was termed Limited Scope PC Human Food inspections which lasted 3-4 days. The FDA has conducted over (as of 1 OCT 2019) over 6760 domestic and 1530 foreign inspections.”
The FDA has also conducted detailed inspection for assessing compliance with Preventive Controls for Human Food and Preventive Controls for Animal Food rules, he says.
“These so-called Full Scope inspections take 4-5 days. The FDA (as of 1 OCT 2019) has conducted over 516 domestic and 150 foreign full scope inspections.”
The FSMA implementation has begun and the FDA is inspecting food plants domestically and internationally with the help of the FDA staff as well the state regulators, notes Vasavada.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
“In addition, the FDA has issued guidance documents to help industry understanding and compliance with the FSMA regulations. The FSPCA is continuing training PCQI/QI for PC Human Food, PC Animal Food and Foreign Supplier Verification Program and have partnered with USDA- Foreign Agriculture Service for training PCQI and Lead Instructors in Mexico, Caribbean, Africa, India and China.”
Adhering to FSMA standards
Wayne Ortner, senior product consultant, FlexiBake, Vancouver, BC, says that FlexiBake software helps [their] customers achieve complete lot traceability and batch tracking from supplier, through production, to distribution.
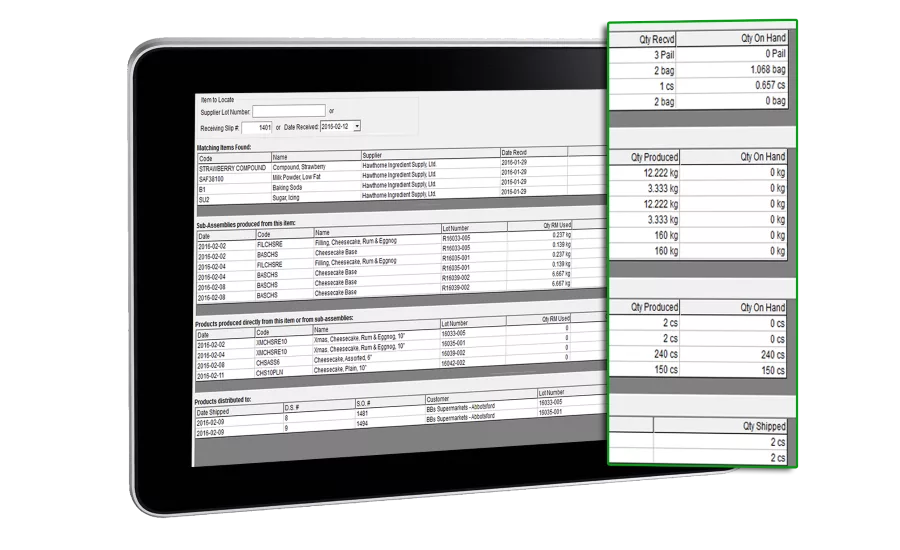
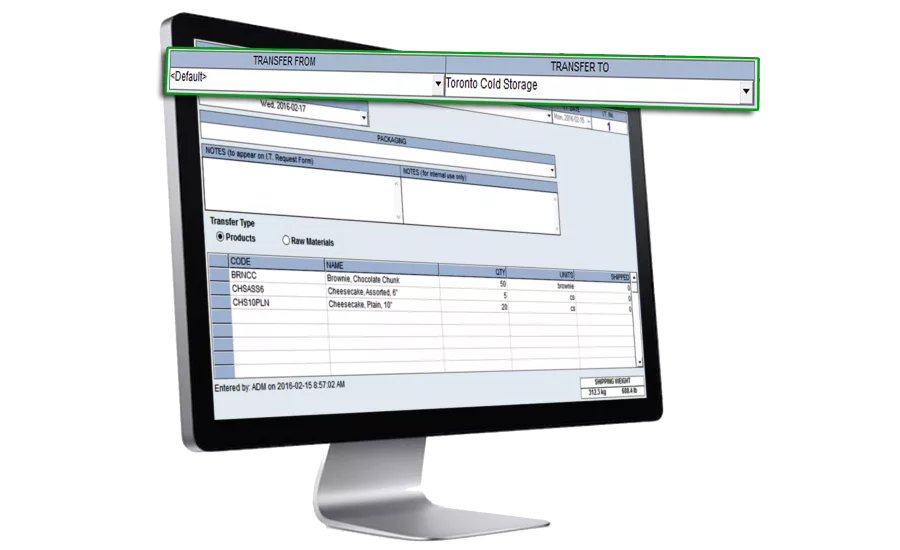
“Using FlexiBake ERP, users can manage inventory in multiple warehouses and production facilities, tracking raw material, work-in-progress, and final product lot numbers during transfers and production. FlexiBake has an instant datamining tool to allow users to search the entire database and report recalls in a matter of seconds.”
FlexiBake’s ability to transfer ingredients and finished product from one location to another means the system can track lot numbers, regardless of where the ingredient was initially delivered to or where the product is being stored prior to final distribution, Ortner says. Best before dates can be automatically generated during production and labels created to manage inventory with longer shelf life.
For distribution networks, FlexiBake’s ability to store and transfer finished product by unique lot numbers means recalls are made easy and more reliable for both the user and the end customer, he explains.
“Users can display lot numbers on labels, pick lists, shipping documents and invoices for their customers. In the event of a recall, information is readily available for the end consumer, the retailer selling the product, the manufacturer producing the product and the supplier of the contaminated ingredients.”
Jesse Juett, senior business development representative, Zipline Logistics, Columbus, OH, says that as a freight broker, they take on the responsibility to communicate FSMA standards between [their] customers, their warehouses, and [their] contracted carriers. They do this by having a handful of protocols that they follow.
“Trailer quality is essential for FSMA compliance. So, we’ve built our network to include food-grade providers. This includes requiring that drivers have trailers that are clean and dry with no holes, leaks, or protruding metal, and that they sweep out their trailer prior to arriving,” he explains.
When delivering food products, drivers are never to open trailer seals, only the receivers, Juett says.
“This is something we overcommunicate and stress to all involved parties. This ensures no tampering can take place and food remains safe from pickup to delivery.”
They also share strict loading requirements and provide partners with a checklist.
“When shipping ingredients, we also ask that drivers have at least two load locks or straps to help secure freight,” Juett adds.
Hitting FSMA benchmarks
“Companies that are involved in the manufacture of food should by now be both fully aware and compliant with each of the FSMA rules that applies to their organization,” says Penny Vyskocil, independent consultant, EAS Consulting Group.
“We have, however, seen that companies that are not involved in the manufacture of food, but solely in the storage and distribution of food, might be lagging a bit behind. The recommended approach to get into compliance as quickly as possible is to align with a GFSI (Global Food Safety Initiative) scheme and adopt these standards into how business is conducted,” she remarks.
“Should a company adopt the GFSI standard for storage and distribution, they will be in good shape to meet FSMA requirements. The standards for storage (warehousing) and distribution are combined into one standard, for ease of applying.”
There are a few options for GFSI schemes to get your organization into a position of compliance, she says:
- BRC (British Retailers Consortium) Standard for Storage and Distribution Issue 3 was published on August 1, 2016 and entered into force on February 1, 2017.
- SQFI (Safe Quality Food Institute’s) SQF Food Safety Code for Storage and Distribution Edition 8 was published in late 2017 and entered into force on January 2, 2018.
- FSSC 22000 also has a Transport and Storage standard.
If warehousing and distribution is where your business is at, another solid food safety resource to investigate and leverage is The Global Cold Chain Alliance, and their core business partners, Vyskocil says. These include: International Association of Refrigerated Warehouses (IARW), World Food Logistics Organization (WFLO), and International Refrigerated Transportation Association (IRTA).
“The Global Cold Chain Alliance is able to come in and conduct a Gap Assessment against current practices and compliance to FSMA Rules for companies operating in the Storage and Transportation segment,” she adds.
Len Steed, manager, HACCP, operations, quality assurance, AIB International, Manhattan, KS, says that for both processors and warehouses, the FSMA benchmarks are whether the “organization has complied with the intent of all applicable FSMA rules and have all requirements been reviewed with documented records to demonstrate effective implementation.”
“The FDA and state inspectors doing FSMA compliance inspections will fully expect that personnel implementing the Food Safety Plan have received documented training for the new regulatory requirements and that personnel performing food safety preventive controls and verification activities understand the importance of those activities to produce and distribute safe food,” he notes.
The Quality Cycle is the tried and true process for effective implementation and continuous improvement is explained as Planning, Performing, Measuring and Improving, Steed says. The Quality Cycle is attributed to the fathers of modern quality typically referred to the Shewhart and/or Deming Quality Cycle.
The obvious question during the planning phase is “Which FSMA requirements apply to our organization?” says Steed.
“If a warehouse is required to register their facility with the FDA under section 415, then the Risk Based Hazard Analysis and Preventive Control rule for Human and/or Animal Food, Mitigation Strategies for Intentional Adulteration and the Sanitary Transport of Human and Animal Food Rule would apply,” he explains.
It is possible that one additional FSMA rule, the Foreign Supplier Verification Program (FSVP) could apply to a warehouse company under certain conditions, he says.
“For example, if a company was buying or agreeing to buy food materials manufactured by a foreign supplier, registered with the FDA and shipping that product to the United States. The designated company responsible for compliance with the FSVP program would be listed as the ‘FSVP’ importer on the prior notice documentation, electronically filed with the Customs and Border Protection (CBP) agency. The FSVP importer would be responsible for reviewing the suppliers of raw materials or food products that have significant hazards referred to as Severe Adverse Health Consequences or Death in Humans and Animals (SAHCODHA) hazards.”
After establishing which FSMA rules apply, the company will need to train “Qualified Individuals” responsible for implementation, review and improvement of the preventive controls, and they will have to receive FDA recognized training for the specific requirements contained in each individual rule, Steed explains.
“The FDA has recognized the Food Safety Preventive Control Alliance (FSPCA) training materials and providers which is coordinated through the Illinois Institute of Technology’s Institute for Food Safety and Health. This standard curriculum is taught by trained Lead Instructors and further information of training offerings can be obtained from the FSPCA website.”
The Preventive Control rule requires implementation by a Preventive Control Qualified Individual (PCQI) who attended a recognized FDA course, such as the Food Safety Preventive Control Alliance (FSPCA) course or is otherwise qualified through education, background and/or experience, he states. The documentation for the Food Safety Plan is as follows:
- A written Hazard Analysis
- Identification of Preventive Controls (PC)
- Documented procedures for the PC activities
- Written Supply Chain Program if applicable
- Written Recall Plan
- Documented Implementation Records
- Corrective Action
- Verification Activities
For warehouses that are handling refrigerated product that require temperature control to prevent pathogen growth or toxin formation, a temperature control program for receipt, storage and shipping refrigerated products should be in place with documentation monitoring for implementation and calibration of devices that are monitoring those temperatures, Steed adds.
“The Mitigation Strategies for Intentional Adulteration (Food Defense) program should be created and implemented by Qualified Individuals. FDA recognized training can be obtained from the FSPCA website.”
The program documentation needs to include the following:
- Documented Food Defense Plan
- Vulnerability assessment to identify significant vulnerabilities and actionable process steps
- Written procedures for monitoring the Mitigation Strategies
- Implementation Records
- Corrective Actions
- Verification Activities
The Sanitary Transport Rule for Human and Animal Food requires that the responsibilities for food safety be defined for the shippers, loaders, receivers and carriers, Steed remarks.
“The requirements include sanitary design of the vehicles, transportation equipment, transportation operations and temperature control where applicable and GMP/personnel hygiene training for employees working in food loading, unloading receivers and carriers. The sanitary requirements for preventing and recognizing potentially unsafe food are documented in procedures and contractual agreements for those companies engaged in transportation and distribution services. It is important to note that this rule does not apply to transportation by ship or air due to the limitations of the law, but all other motorized vehicles such as tractor trailer and rail are covered under this rule to ensure food safety.”
The FSPCA does not have an FDA training course for this rule but an hour-long training module for carriers is available from the FDA at their website, says Steed. There are additional regulatory requirements applicable to the transportation of food, which should be reviewed as applicable to the company’s operations and distribution network, e.g. 21 CFR parts 117 (human food), 118 (shelled egg), 225 (medicated feed), 507 (animal food) and 589 (prohibited substances in animal feeds).
After FSMA training
After personnel have been trained, documented procedures for “best practices” have been developed and job-specific training for personnel performing food safety and inspection requirements completed, then the company will be ready for internal and third party audits to judge the effectiveness of implementation, says Steed.
“The personnel performing the audit and inspection function for the company must be ‘Qualified Auditors’ to perform this function. The audits will provide feedback on effective implementation through direct observation of activities, record reviews and interviews of personnel performing and supervising these activities. It is not unusual to find the need for corrective action when implementing new or revised procedures for loading, unloading, temperature checks, vehicle inspection/cleaning records and seal checks,” he remarks.
Documenting the corrective actions during the implementation phase provides the company with an opportunity to take credit for documented improvements that were made to prevent reoccurrence of work activity performance or a recordkeeping issue, Steed says.
“The FDA has stated that their inspections will report on the effectiveness of implementation so being able to show where deficiencies were observed and corrected will serve as evidence of effective corrective action and implementation. The FDA and state have the right to see any documented procedures, records and request copies of records associated with food safety. Having a plan and preparing for these more rigorous audits is not only advisable, but serves as a good crisis management exercise.”









