A Review of Outbreak Investigations of Salmonella Infections Associated with Cashews and Cashew-Containing Food Products in the U.S.
Salmonella spp. have long been associated with low-moisture foods such as nuts and nut-derived products

Over the past 40 years, consumption of cashews and other nuts in the U.S. has markedly increased, due to their nutritional properties and perceived health benefits.1,2 Plant-based dairy food alternatives are also increasing in popularity and are capturing a small but robust part of the global food market. In the U.S., retail sales of plant-based milk and dairy alternatives totaled $2.6 and $2.1 billion dollars, respectively, in 2021. Annual retail sales of plant-based cheese alternatives increased 85 percent from 2018 to 2021.3 This increase in popularity has led to a variety of plant-based alternative foods being available to consumers, including those made from almonds, soy, coconut, oats, and emerging products made of lupine and cashews.4,5 Currently, the U.S. has not established compositional requirements for any nut-based milk alternatives or nut-based cheese alternatives. However, in February 2023, FDA published a draft guidance on the labeling of plant-based milk alternatives and the use of voluntary nutrient statements.6
Nut-based milk alternatives are generally made from liquid-based extracts of plant materials. In addition to water and plant extracts, products may contain other ingredients such as sweeteners, salt, oils, functional ingredients, flavorings, and vitamin and mineral fortifications.7 The fluid obtained from the breakdown of nuts soaked in water and homogenized is similar to the appearance of cow's milk.5,8 Nut-based cheese alternatives are also products made from the liquid extracts of nuts with the addition of other ingredients. They are shaped and may be processed similarly to dairy milk-based cheese, such as by fermentation.9
Salmonella spp. have long been associated with low-moisture foods such as nuts and nut-derived products, with varying prevalence (0.20–0.55 percent) observed in tree nuts, including cashews, at retail.10,11,12 Virtually all cashews are imported to the U.S., with the top sources of cashew nuts in 2020 being Vietnam, India, and Brazil.13 Some nuts, including cashews, are imported as a "raw" product (i.e., one that has not been subjected to a process to adequately reduce pathogens, such as a kill step). If a kill step is not applied in the U.S., it can result in the consumption of raw nuts or nut products. Salmonella contamination of such products has resulted in several notable outbreaks in the U.S. in the past ten years, including those in which cashew-containing products have been implicated as a vehicle.14,15,16
The U.S. Food and Drug Administration (FDA), specifically the Center for Food Safety and Applied Nutrition's (CFSAN's) Coordinated Outbreak Response and Evaluation (CORE) Network, in collaboration with the Centers for Disease Control and Prevention (CDC) and state and local public health and regulatory partners, investigates foodborne illness outbreaks, including those caused by Salmonella spp. Although most people recover without treatment, as of 2021, CDC estimated that over 26,000 people are hospitalized and approximately 420 persons die each year in the U.S. from acute salmonellosis.17 These consequences underscore the importance of applying appropriate food safety practices when producing new plant-based alternatives to control the presence of, and prevent their contamination with, Salmonella spp.
This article presents a brief overview of outbreak investigations of Salmonella infections linked to the consumption of cashews and cashew-containing food products. It also presents the challenges encountered, lessons learned, and relevant regulatory requirements for importers and manufacturers of cashews and cashew-containing products.
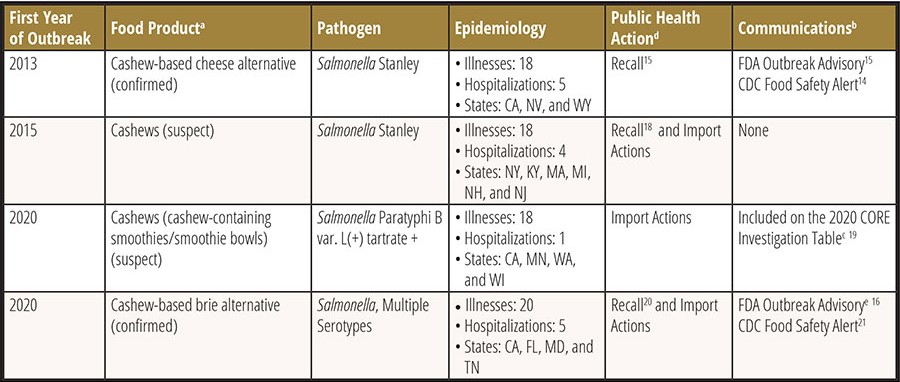
Overview of Foodborne Illness Outbreaks Associated with Cashews and Cashew-Containing Products
Strong and Unique Epidemiologic Signal Cracks the Case
In late 2013, FDA, CDC, and state and local partners began investigating an outbreak of Salmonella Stanley infections with ill persons reporting consumption of a specific brand of non-dairy cheese alternative made from ground cashews and additional ingredients (Table 1).15 Nine of 12 (75 percent) ill persons interviewed reported eating the cashew-based cheese alternative in the week before becoming ill. All of the ill persons reported that they had eaten the same specific brand or had purchased it at a retailer.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
"There was confidence in the epidemiologic information since it was pretty conclusive and helped steer the investigation in the right direction to ultimately protect public health," explained Ashley Grant, an Epidemiologist with FDA CORE.
Since this specific product was made in California, the California Department of Public Health (CDPH) conducted a traceback investigation and determined that the cashews were imported. CDPH collected two samples of the product, an opened consumer sample of cashew-based cheese alternative and a sample collected at the manufacturer that was identified as "fermenting cashews"—a mix of cashews, municipal water, and dietary supplement. Both samples were positive, with the consumer sample yielding the outbreak strain, matching by pulsed-field gel electrophoresis, and the "fermenting cashew" sample yielding Salmonella Weltevreden.
Investigators observed that the cashews used were labeled as "raw, shelled," indicating that a process to adequately reduce pathogens (i.e., a "kill step") was not applied prior to import. The manufacturer relied on fermentation to control foodborne pathogens, but the manufacturer was using a dietary supplement instead of a true starter culture to initiate the fermentation. The intended fermentation process and the desired microbial changes to enhance safety may not have occurred due to the use of this dietary supplement. CDPH warned the public not to eat the implicated cashew-based cheese alternative product, and the manufacturer issued a voluntary recall of its cashew-based cheese alternative product with expiration dates on or before April 19, 2014.
The root cause of this outbreak (i.e., the underlying cause of contamination of the product) could not be determined. Although investigators observed that the cashews used were labeled as "raw, shelled," and that no process to adequately reduce pathogens (i.e., a "kill step") was applied during production, contamination from the production environment or from another incoming ingredient could not be ruled out. As of March 31, 2014, a total of 18 ill persons were reported in California (16), Nevada (1), and Wyoming (1).
Testing Imported Products as a Tool to Protect Public Health
In March 2015, FDA, CDC, and state and local partners began investigating an outbreak of Salmonella Stanley infections with 11 of 18 ill persons (61 percent) reporting consuming cashews; state partners from the Massachusetts Department of Public Health and the New York State Department of Agriculture and Markets sampled cashews from retail stores, ill persons' homes, and a retail smoothie beverage containing blended cashews, but all samples were negative for Salmonella spp.
FDA conducted a traceback investigation and determined that imported cashews consumed by ill persons were provided by three firms in two countries; one of the two exporting countries was implicated as the source of this outbreak. Additionally, cashews labeled as "raw" from firms located in the implicated country tested positive for a non-outbreak strain of Salmonella. As a result, FDA initiated more thorough testing of cashews imported by the three firms.22 The importer of the cashews from the two firms located in this country recalled the positive product lot still on the market. Investigators were not able to determine a root cause for the outbreak, but they did identify firms for product screening to help prevent contaminated future product from entering the U.S. market.
"This was one of those outbreaks where we took import actions to make sure that contaminated food was not entering the U.S. market. It would have been ideal to determine the source and route of contamination, but we did not have enough information to do so," added Johnson Nsubuga, Lead Coordinator for the response efforts of this outbreak investigation with FDA CORE.
As of October 6, 2015, 18 ill persons were reported in six states: New York (10), Kentucky (2), Massachusetts (2), Michigan (2), New Hampshire (1), and New Jersey (1).
Common Retailer Helps Narrow Down Suspect Ingredients
In October 2020, FDA, CDC, and state and local partners began investigating an outbreak of Salmonella Paratyphi B var. L(+) tartrate + infections. Ill persons reported consuming menu items from multiple locations of a chain juice bar.23 FDA and state partners conducted a traceback investigation of the four ingredients most commonly reported by nine ill persons who consumed product at multiple retail locations. Cashew-based milk alternative was the most commonly reported ingredient and became the leading hypothesis.
The juice bars produced their cashew-based milk alternative using a typical process for at-home cashew milk, which involves soaking cashew pieces in water under refrigeration, adding flavoring, and then blending all ingredients together, sometimes with ice.24,25,26 The sole supplier of cashew pieces during the timeframe of interest had previously been subject to import actions. The imported cashews were labeled as "raw," and there was no indication that they had been subjected to a kill step. State and local officials collected samples at several retail locations; however, no positive samples were detected, and the investigation was unable to determine a root cause for the outbreak.
"Multi-ingredient foods always pose an added challenge to our investigations, and this was no different," stated Tami Cloyd, a Veterinarian with FDA CORE.
As of December 2020, 18 ill persons were reported across four states: Minnesota (9), California (6), Washington (2), and Wisconsin (1); one person was hospitalized.
Unique Exposure Information Helps Solve the Outbreak Again
In April 2021, FDA, CDC, and state and local partners began investigating an outbreak of Salmonella Duisburg infections, but several serovars would eventually be associated with this outbreak.16
"This outbreak had a unique signal," Jennifer Beal, an Epidemiologist with FDA CORE, explained. "Early exposure information indicated a cashew-based brie alternative produced by a single domestic firm as an item of interest."
State partners from the CDPH and the Tennessee Department of Health collected intact retail samples of cashew-based brie alternative products (each state sampled different varieties) produced by the firm of interest and an open consumer product sample. Six of these samples—five from California (four intact and one open product) and one from Tennessee—yielded matches to one of the outbreak strains and, in some cases, other clinicals. FDA conducted an inspection of the manufacturer's facility, and several samples of cashews, in-process product (aging cashew-based brie), and environmental surfaces were positive for various Salmonella serovars. As a result, the manufacturer conducted a voluntary recall of all of its cashew-based products.
FDA also conducted an inspection of the cashew importer and verified the corrective actions taken by the importer to ensure it was in compliance with the Foreign Supplier Verification Program (FSVP). The contaminated imported cashew pieces were the likely source of Salmonella. A total of 20 ill persons were reported across four states: California (15), Florida (2), Maryland (1), and Tennessee (2). Seven people were infected with Salmonella Typhimurium, five people were infected with Salmonella Duisburg, four people were infected with Salmonella Urbana, and four people were infected with Salmonella Chester.
Regulatory Requirements for Facilities and Importers with Respect to Cashews and Cashew-Based Products
Federal Food, Drug, and Cosmetic Act
Cashews are a raw agricultural commodity subject to the adulteration provisions of the Federal Food, Drug, and Cosmetic Act. However, they are not covered by the "Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption" [21 CFR part 112; the Produce Safety Rule (PSR)]. While the PSR established science-based minimum standards for the safe growing, harvesting, packing, and holding of fruits and vegetables grown for human consumption, certain produce is excluded from the rule. For example, the rule does not cover produce that (1) is grown for personal or on-farm consumption, (2) is not a "raw agricultural commodity," or (3) is identified as rarely consumed raw (RCR). Cashews are classified as RCR, based on nationally representative consumption pattern data [21 CFR 112.2(a)(1)]. Commodities are classified as RCR because they are almost always (but not necessarily never) consumed after being cooked; cooking is a kill step that can be expected to adequately reduce the presence of pathogens, in most cases.27
The Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food Rule
The Current Good Manufacturing Practice (CGMP), Hazard Analysis, and Risk-Based Preventive Controls for Human Food rule (21 CFR part 117; the CGMP & PC rule) applies to facilities that must register with FDA that are foreign, as well as domestic facilities engaged in the manufacturing/processing, packing, or holding of food, including processed cashews or cashew-based products, for consumption in the U.S. Among other activities, the regulation requires facilities to conduct a hazard analysis and implement preventive controls (i.e., process, sanitation, allergen, supply chain) and preventive controls management components for hazards identified as requiring a preventive control (see 21 CFR 117 subpart C), unless subject to an exemption (see 21 CFR § 117.5 regarding Exemptions).
It is worth noting that the initiation of compliance dates for subpart C ranged between 2016 and 2018 for most of the preventive controls provisions of the PC rule, depending on the firm size, and two of the outbreaks occurred before that time. The CGMP requirements address such topics as personnel, plant and grounds, equipment and utensils, sanitary operations and facilities, processes and controls, and warehousing and distribution, all of which are important in minimizing and/or preventing contamination of food.
Foreign Supplier Verification Program (FSVP) for Food Importers Rule
Unless exempt or subject to modified requirements, importers must verify that the food they import, such as cashews, meets U.S. safety standards. Importers are responsible for actions that include determining known or reasonably foreseeable hazards; evaluating the risk posed by a food based on the hazard analysis and the foreign supplier's performance; using the evaluation of the risk posed by an imported food and the supplier's performance to approve suppliers and determine appropriate supplier verification activities; and conducting supplier verification activities.
Importers have the flexibility to tailor supplier verification activities to unique food risks and supplier characteristics. Verification activities may include an annual onsite audit of the supplier's facility, sampling and testing, and/or a review of the supplier's relevant food safety records. In addition, importers are required to take corrective actions in certain circumstances. The appropriate corrective measure will depend on the circumstances, but one example is discontinuing the use of a foreign supplier until the cause of noncompliance, adulteration, or misbranding has been adequately addressed.
Challenges Encountered During Outbreaks and Lessons Learned
Processed cashew products are relatively new to the U.S. market and, with the increasing popularity of plant-based food products, it is expected that new processes and producers will enter the market. Among the firms linked to outbreaks, there was a general lack of knowledge around basic food safety practices and considerations, such as Salmonella spp. being a reasonably foreseeable hazard in tree nuts, and/or applicable regulations. For example, when cashews were soaked and/or fermented during manufacturing, inspectors noted that firms seemed unaware of the possible risk of pathogen growth during this process. Additionally, the microbial and chemical changes that cashews undergo during fermentation or aging are not well understood and, as a result, questions remain regarding the adequacy of these processes to significantly minimize or prevent pathogen growth.
"One of the things that becomes immediately clear from reviewing past outbreak investigations linked to cashews and cashew-based products is the fact that cashews, as an ingredient, were imported for all four outbreaks," noted Tami Cloyd at FDA CORE.
Appropriate controls for the biological hazards that may have been present in the RCR cashews were not implemented by the firms that produced cashew-containing products. Importers may not have applied a disclosure to the product, stating that the cashews had not been treated to control pathogens, with one importer supplying cashews during three outbreaks and a separate importer supplying cashews during the fourth outbreak. As there is no known domestic production of cashews,28,29 it is particularly important for U.S. importers and manufacturers to be knowledgeable about how imported cashews are labeled and treated. Appropriate actions could include using the FSVP to import only cashews treated with a kill step or leveraging the CGMP & PC rule within importers' own process.
Communication challenges arose with manufacturing facilities and importers during several outbreak investigations. Some records and product documentation from importers were provided in languages other than English, which stalled the investigation. Furthermore, inadequate communication between implicated firms, such as between processors, retailers, and their suppliers, may be a contributing factor in failing to control for contamination. During the 2021 outbreak linked to cashew-based brie alternative products, FDA noted that the importer sold both cashews treated with a kill step and cashews that were not treated with a kill step, but the records associated with product purchasing made this distinction unclear to the manufacturer. This lack of clarity between suppliers and producers in the cashew supply chain is one factor that could result in the failure to implement adequate preventive controls during production of ready-to-eat (RTE) foods.
The organoleptic characteristics of cashews have helped establish them as a desirable ingredient for dairy alternative foods, ones that may be consumed without a kill step.30,31 None of the inspected firms implicated in these outbreaks implemented a kill step, the absence of which increased the risk of Salmonella persistence and growth when raw cashews were used as an ingredient. With the increasing popularity and production of these products, establishing and implementing adequate controls to significantly minimize or prevent pathogen growth will be imperative to reduce the likelihood of foodborne illness associated with their consumption. Furthermore, some of the firms involved in these outbreaks were relatively small and, as a result, may have had limited resources dedicated to establishing and implementing controls to significantly minimize or prevent pathogens in their products. Some may not have realized that raw cashews could contain pathogens such as Salmonella. However, when manufacturers produce RTE products using cashews, they must establish and implement adequate controls for pathogens that may be present on those ingredients.
Additionally, although cashews are exempt from the PSR, growers, harvesters, packers, and holders of cashews may want to consult this rule for potential mitigation strategies to reduce risk of contamination. Treatments such as steam and/or chemical fumigants are examples of control measures that manufacturers of cashew-containing products can implement to adequately reduce the presence of pathogens including Salmonella.32 Regardless of firm size, and regardless of the regulatory status of the manufacturers or ingredients, the statutory prohibition against the introduction or delivery for introduction into interstate commerce of adulterated food [section 301(a) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 331(a)] applies. Under section 402(a)(1) of the FD&C Act [21 U.S.C. 342(a)(1)], a food is deemed adulterated if it bears or contains any poisonous or deleterious substance that may render it injurious to health. Under section 402(a)(4) of the FD&C Act [21 U.S.C. 342(a)(4)], a food is deemed to be adulterated if it has been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with filth, or whereby it may have been rendered injurious to health.
Conclusions
While definitive sources of contamination were not identified during these outbreak investigations, adequate controls for biological hazards including, but not limited to, application of a kill step or supplier controls to ensure non-use of raw cashews, were not implemented by the cashew processors. It is important for importers and processors to comply with existing requirements. Additional studies11,12 evaluating Salmonella prevalence and levels in the imported cashew supply and persistence during the production of cashew-based products could inform prevention strategies, such as targeted outreach to industry, to decrease the potential for foodborne illnesses associated with these products.
Acknowledgments
The response efforts to these outbreaks included public health officials at local and state agriculture and health departments in the U.S., who serve as the backbone of multistate outbreak investigations. The assistance of state partners, including Rapid Response Teams, was crucial in the collection and analysis of product samples, traceback documents, and epidemiologic information. The review and input of subject matter experts from FDA CFSAN's Office of Food Safety, Office of Compliance, and Office of Chief Counsel are appreciated.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the Food and Drug Administration (FDA).
References
- Ogunwolu, S. O., F. O. Henshaw, H.-P. Mock, A. Santros, and S. O. Awonorin. "Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut." Food Chemistry 115 (2009): 852–858.
- U.S. Department of Agriculture Economic Research Service (USDA ERS). "Almonds lead increase in tree nut consumption." May 31, 2019. https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=93152.
- Ignaszewski, E. "2021 U.S. Retail Market Insights, Plant-based Foods." 2022. https://gfi.org/wp-content/uploads/2022/03/2021-U.S.-retail-market-insights_Plant-based-foods-GFI.pdf.
- Chalupa-Krebzdak, S., C. J. Long, and B. M. Bohrer. "Nutrient density and nutritional value of milk and plant-based milk alternatives." International Dairy Journal 87 (2018): 84–92.
- Sethi, S., S. K. Tyagi, and R. K. Anurag. "Plant-based milk alternatives an emerging segment of functional beverages: A review." Journal of Food Science and Technology 53 (2016): 3408–3423.
- U.S. Food and Drug Administration (FDA). Draft Guidance for Industry: Labeling of Plant-Based Milk Alternatives and Voluntary Nutrient Statements. February 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-labeling-plant-based-milk-alternatives-and-voluntary-nutrient-statements.
- McClements, D. J., E. Newman, and I. F. McClements. "Plant-Based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance." Comprehensive Reviews in Food Science and Food Safety 18 (2019): 2047–2067.
- Oyeyinka, A. T., J. O. Odukoya, and Y. S. Adebayo. "Nutritional composition and consumer acceptability of cheese analog from soy and cashew nut milk." Journal of Food Processing and Preservation 43 (2019): e14285.
- British Columbia Centre for Disease Control. "Food Issue: Notes from the field: Fermented nut cheese." August 16, 2017. http://www.bccdc.ca/resource-gallery/Documents/Educational%20Materials/EH/FPS/Food/Fermented_Nut_Cheese.pdf.
- Little, C. L., N. Rawal, E. de Pinna, and J. McLauchlin. "Survey of Salmonella contamination of edible nut kernels on retail sale in the UK. Food Microbiology 27 (2010): 171–174.
- Zhang, G., L. Hu, Y. Luo, S. M. Santillana Farakos, R. Johnson, V. N. Scott, P. Curry, D. Melka, E. W. Brown, and E. Strain. "Survey of Salmonella in raw tree nuts at retail in the United States." Journal of Food Science 86 (2021): 495–504.
- Zhang, G., L. Hu, D. Melka, H. Wang, A. Laasri, E. W. Brown, E. Strain, M. Allard, V. K. Bunning, S. M. Musser, R. Johnson, S. M. S. Farakos, V. N. Scott, R. Pouillot, J. M. V. Doren, and T. S. Hammack. "Prevalence of Salmonella in Cashews, Hazelnuts, Macadamia Nuts, Pecans, Pine Nuts, and Walnuts in the United States." Journal of Food Protection 80 (2017): 459–466.
- USDA ERS. "Fruit and Tree Nuts Data: Market Segment." 2022. https://data.ers.usda.gov/reports.aspx?programArea=fruit&groupName=Tree%20nuts&ms_key=F&ID=17887#Pae394dff610c470e85e3e9e5e231d4a7_3_613iT0R0R0x2.
- U.S. Centers for Disease Control and Prevention (CDC). "Multistate Outbreak of Salmonella Stanley Infections Linked to Raw Cashew Cheese (Final Update)." January 31, 2014. https://www.cdc.gov/salmonella/stanley-01-14/index.html.
- FDA. "FDA Investigates Outbreak of Salmonella Stanley Linked to Cashew Cheese from The Cultured Kitchen®." January 3, 2014. http://wayback.archive-it.org/7993/20171114154920/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm380307.htm.
- FDA. "Outbreak Investigation of Salmonella: Jule's Cashew Brie (April 2021)." July 7, 2021. https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-jules-cashew-brie-april-2021.
- CDC. "Salmonella." 2022. https://www.cdc.gov/salmonella/index.html.
- Food Safety News. "Vietnamese Cashews Recalled Due to Possible Salmonella Contamination." June 1, 2015. https://www.foodsafetynews.com/2015/06/vietnamese-cashews-recalled-due-to-possible-salmonella-contamination/#more-112368.
- FDA. "About the CORE Network." January 25, 2023. https://www.fda.gov/food/outbreaks-foodborne-illness/about-core-network.
- FDA. "Jule’s Foods Issues Voluntary Recall of Jule’s Foods Products Because of Possible Health Risk." April 22, 2021. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/jules-foods-issues-voluntary-recall-jules-foods-products-because-possible-health-risk.
- CDC. "Salmonella Outbreak Linked to Jule’s Cashew Brie." July 7, 2021. https://www.cdc.gov/salmonella/duisburg-04-21/index.html.
- FDA. "Import Alert 99-19. June 8, 2023. https://www.accessdata.fda.gov/cms_ia/importalert_263.html.
- Minnesota Department of Health. "Health officials investigate Salmonella cases linked to NéktƏr Juice Bar in Woodbury. October 9, 2020. https://www.health.state.mn.us/news/pressrel/2020/salmonella100920.html.
- Food Network. "Almond or Cashew Milk." 2022. https://www.foodnetwork.com/recipes/almond-or-cashew-milk-3362240.
- Perry, D. "Basic Nut Milk." December 17, 2013. https://www.bonappetit.com/recipe/basic-nut-milk.
- Uskokovic, S. "Homemade Cashew Milk." December 27, 2021. https://www.thekitchn.com/cashew-milk-recipe-23255672.
- FDA. "FDA Fact Sheet: Produce Safety Rule (21 CFR 112). 2018. https://www.fda.gov/files/food/published/FDA-Fact-Sheet-Produce-Safety-Rule-21-CFR-112-Rarely-Consumed-Raw-Produce-PDF.pdf.
- McLaughlin, J., C. F. Balerdi, and J. Crane. "Cashew-apple fruit growing in the Florida home landscape." 2008. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=54406868d8d1c4eced49a0a8c383b86c4d0360c0.
- USDA National Agricultural Statistics Service Information (NASS). "Agricultural Statistics 2020." 2020. https://downloads.usda.library.cornell.edu/usda-esmis/files/j3860694x/z890sn81j/cv43pq78m/Ag_Stats_2020_Complete_Publication.pdf.
- Pua, A., V. C. Y. Tang, R. M. V. Goh, J. Sun, B. Lassabliere, and S. Q. Liu. "Ingredients, Processing, and Fermentation: Addressing the Organoleptic Boundaries of Plant-Based Dairy Analogues." Foods 11 (2022).
- Short, E. C., A. J. Kinchla, and A. A. Nolden. "Plant-Based Cheeses: A Systematic Review of Sensory Evaluation Studies and Strategies to Increase Consumer Acceptance." Foods 10 (2021).
- Saunders, T., J. Wu, R. C. Williams, H. Huang, and M. A. Ponder. "Inactivation of Salmonella and Surrogate Bacteria on Cashews and Macadamia Nuts Exposed to Commercial Propylene Oxide Processing Conditions." Journal of Food Protection 81 (2018): 417–423.
Margaret Kirchner, Ph.D., is a Microbiologist at the U.S. Food and Drug Administration (FDA).
Gordon R. Davidson, Ph.D., is a Biologist at FDA.
Spencer Carran, Ph.D., is a Biologist at FDA.
Julia Mangia, M.P.H., is a Health Communications Specialist with the FDA CORE Network.
Andrea J. Krause, Ph.D., is a Food Technologist at FDA.
Karunya Manikonda, M.P.H., is an Epidemiologist at the Centers for Disease Control and Prevention (CDC).
Zachary D. McCormic, M.P.H., is an Epidemiologist at the Division of Foodborne, Waterborne, and Environmental Diseases at CDC's National Center for Emerging Zoonotic and Infectious Diseases.
Laura Gieraltowski, Ph.D., is Team Lead for the Foodborne Outbreak Response Team at CDC.
Katherine E. Marshall, M.P.H., is Deputy Lead of the Outbreak Response and Prevention Branch of CDC.
Stelios Viazis, Ph.D., is a Consumer Safety Officer at FDA.






