Food Safety Insights: 5 Years Later, Part 2
Celebrating the Food Safety Insights partnership with Food Safety Magazine

In the last issue of Food Safety Magazine,1 we revisited some of the topics that we have covered in this column since it was first published in the February/March 2017 issue. Last month, we looked at many of the testing-related topics that we have covered. This month, we will look at a few of the other impactful subjects that have made an appearance here and had an impact on our markets in these past 5 years.
Food Safety Modernization Act (FSMA)FSMA was certainly on everyone’s mind over these past years and made many appearances in Food Safety Insights. FSMA was signed into law in 2011, but major compliance dates really started to occur starting in late 2016, which conveniently coincided with the start of this column in early 2017. FSMA focused everyone’s attention more on food safety and practices, the switch from Hazard Analysis and Critical Control Points to Hazard Analysis and Risk-Based Preventive Controls, more aggressive U.S. Food and Drug Administration (FDA) inspections, supply chains and the Foreign Supplier Verification Program, and an overall reevaluation of food safety practices.
It even drove an internal review of the inspection and regulatory practices of FDA itself. FDA signaled its desire to become more of a resource to food processors, with what many people described as the transition from the “food safety police” to partnering with food processors with their stated goal to “educate before we regulate.” We, of course, saw this as an invitation to find out if processors believed this was possible. In 2017, we asked processors, “Does FDA educate before it regulates?”2 In that first survey, the opinion ran against FDA by a rate of 60 percent disagree to 40 percent agree (Figure 1). In that same article, we promised to revisit this question periodically to continue to gauge progress.
In 2019, we did just that; that survey showed a significant reversal, with 72 percent agreeing that FDA educates first.3 We heard from many people at the time that they saw a significant change in the posture of the agency, with inspectors focusing more on cooperation to improve outcomes than on just writing violations. At least one person at the agency was pleased with this 2-year reversal of opinion as the article garnered a retweet from FDA’s deputy commissioner for food policy and response, Frank Yiannas (thanks, Frank!).
FIGURE 1. Do you believe FDA educates before it regulates?
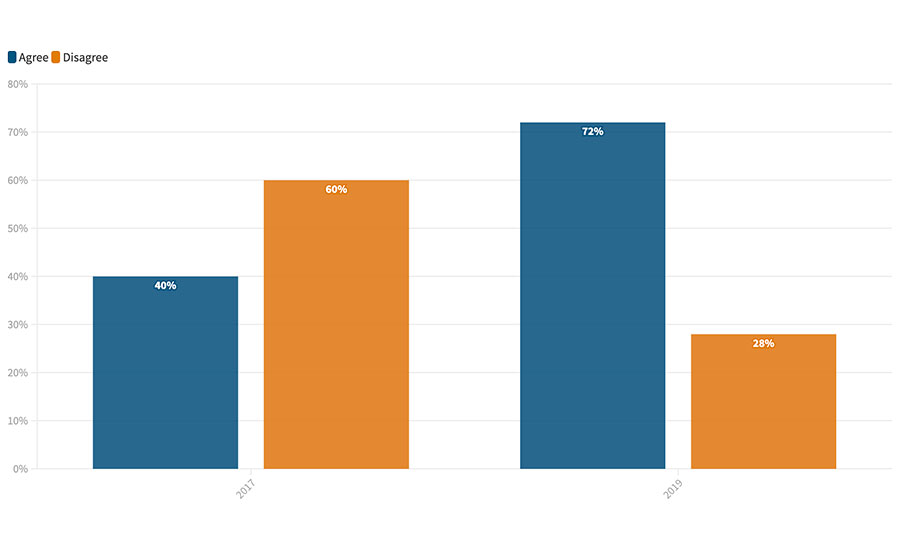
COVID-19
No discussion of the past 5 years would be complete without some mention of COVID-19. Volumes have been written about the impact the pandemic had—and continues to have—on food production, demand, and supply chains. There is no need to reiterate those details, but it is important to understand the midterm effect that recovery from the pandemic will have and also the long-term impact of changes that will become permanent. The onset of the pandemic significantly disrupted the market as demand dramatically shifted away from restaurants and food service to more home meals, causing commensurate disruptions in supply and production in essentially every area of the market. As we continue to recover, the shift back to a pre-pandemic market will also create disruptions as demand probably shifts faster than supply chains can adjust, causing short-term shortages but also an opportunity for suppliers if they can keep up with demand. We expect to see above-average growth rates in all areas of food safety testing materials and supplies over the next year or so and likely into 2023.
Supply Chain
Some areas of supply chains have most likely been changed forever. When we asked about pandemic and supply chain issues in our October/November 2020 Food Safety Insights,4 we heard that the shortages and disruptions throughout the supply chain were being seen as not just an area for supply and logistics professionals but also as a fundamental issue of business continuance. This was causing many to reevaluate their processes due to lessons learned from the pandemic (Figure 2).
FIGURE 2. Have you and your organization been reevaluating your supplier verification processes due to lessons learned from the pandemic?
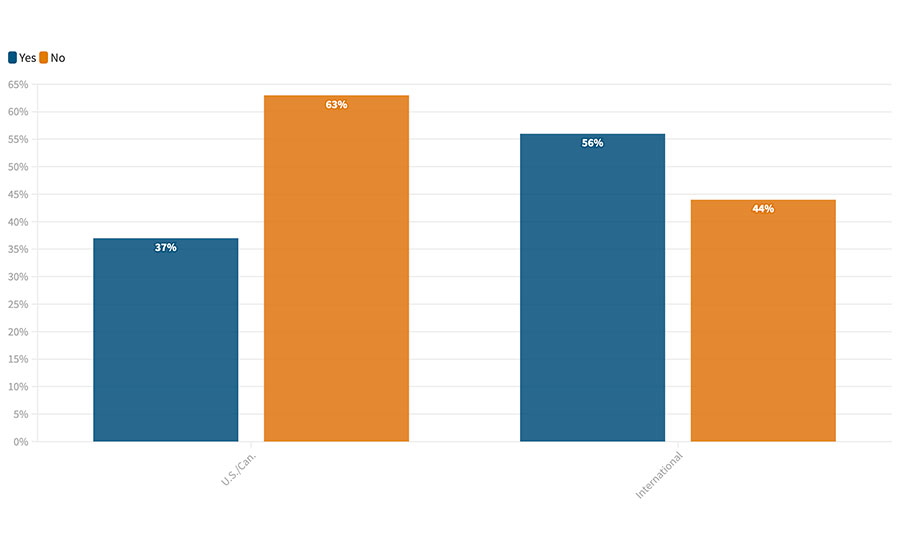
Food processors with whom we spoke said that COVID-related shutdowns of their primary suppliers, and their previous reluctance to qualify secondary and tertiary suppliers, threatened not only their ability to produce any product but also, for many, their ability to survive the pandemic and remain in business. To put this another way, processors reported that they realized that they had become “too lean” and that a focus on supply chain cost efficiency and “just-in-time” resupplying had taken out any of the slack they had in their system and denied them the time they needed to respond. This was too close a call for many, and the discussion has moved from “just-in-time” to “just-in-case” to build more flexibility into the supply chain and embrace a philosophy that the cost of some amount of redundancy and maintenance of alternate suppliers is money well-spent as a hedge against future market disruptions. As we mentioned in earlier articles about in-house sample analysis, it seems that issues of risk, security, and continuity are assuming a bigger role in business decision-making in food processing rather than a singular focus on costs.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
Other changes that we expect to see in supply chain management may include maintaining the use of virtual inspections. The use of remote technology for audits and inspections increased during the pandemic to keep inspectors and the employees at the production site safe by reducing the need for visitors. It should also be noted that local health departments conduct many regulatory inspections. The inspectors, as public health officials acutely aware of the risks, certainly wanted to protect themselves from exposure but also to make sure they were not contributing to the spread of infection by frequently traveling from site to site.
But with the likely need to expand the number of suppliers, and especially the focus on international suppliers, remote cameras and virtual inspection techniques will become even more valuable to make the additional site visits easier, safer, and less expensive to inspect. They may also make it easier to facilitate better relationships with these suppliers. Several people have pointed out that being able to visit a supplier without expensive travel will make more frequent contacts possible. And these contacts will not only have to be made during a formal audit but as informal check-ins to stay on top of suppliers’ processes and compliance efforts. It has also been pointed out to us that virtual visits may also facilitate more participation from higher-level executives than previously would have been practical. A supply chain vice president, for example, may be able to visit only a few suppliers each year if she has to travel to each one. This would be particularly true for international supply chains. But with virtual visits, that vice president can get a “firsthand” look at, and a better understanding of, many more of her suppliers’ operations and staff. So, the necessity caused by COVID may have produced a useful dividend in improving supply chain control.
Over the past 5 years, we have also covered allergen control, food fraud, sanitation, food safety investments, your opinion on your career in food safety, and many other topics. And while I routinely make market forecasts in this column, I do not claim to know what the future will bring. What I do know is that it will be interesting and impactful on all our businesses, and we look forward to covering as much of it as we can in Food Safety Magazine and here in Food Safety Insights.
References
- https://www.food-safety.com/articles/7305-food-safety-insights-5-years-later-part-1.
- https://www.food-safety.com/articles/5319-what-industry-and-fda-are-thinking-about-fsma-implementation.
- https://www.food-safety.com/articles/6176-what-industry-and-fda-are-thinking-about-fsma-implementation-part-1.
- https://www.food-safety.com/articles/6808-food-supply-chains-and-covid-19-impacts.
Bob Ferguson is president of Strategic Consulting Inc. and can be reached at bobferguson9806@gmail.com or on Twitter at @SCI_Ferguson.









