Estimating the Cost of Environmental Monitoring

Many of the small business owners and operators with whom I interact who manufacture ready-to-eat (RTE) foods and whose facilities are subject to Subparts C & G of the Preventive Controls for Human Food rule are surprised to find out that the U.S. Food and Drug Administration (FDA) expects them to conduct environmental monitoring. I write “expect” because the requirement is conditional on two things: (1) the hazard evaluation includes an evaluation of environmental pathogens and (2) a determination is made that an environmental pathogen (e.g., Listeria monocytogenes) constitutes a hazard requiring a preventive control.
Hazard Evaluation
If the food being manufactured is a food (e.g., salsa) that is packaged and then subjected to a lethal treatment, environmental monitoring would not be required by the Preventive Controls for Human Food rule.
However, if the salsa is RTE, exposed to the environment prior to packaging, and does not receive a lethal heat treatment or other control measure (such as a formulation lethal to the pathogen) that would significantly minimize the pathogen, then the manufacturer would have to include an evaluation of environmental pathogens in its hazard evaluation [21 C.F.R. §117.130(c)(1)(ii)].
Environmental Pathogen Is Determined to be a Hazard Requiring a Preventive Control
If in the course of the hazard evaluation, the manufacturer (e.g., a salsa manufacturer) determines that an environmental pathogen (such as L. monocytogenes or “Lm”) constitutes a hazard requiring a preventive control, FDA is explicit that the appropriate preventive control would be a sanitation preventive control [21 C.F.R. §117.135(c)(3)]. In such circumstances, the manufacturer is required to conduct environmental monitoring in order to verify that the preventive control is consistently implemented and effectively and significantly minimizing or preventing the hazard [21 C.F.R. §117.165(a)(3)].
If in the course of the hazard evaluation, the manufacturer determines that an environmental pathogen does not constitute a hazard requiring a preventive control when FDA regulations and guidance indicate they should, the manufacturer would need to back up the determination with scientific justification. In most cases, a manufacturer would be unlikely to persuade FDA that an environmental pathogen is not a hazard requiring a preventive control if the food is: (a) RTE, (b) exposed to the environment prior to packaging, and (c) does not receive a lethal heat treatment or other control measure (such as a formulation lethal to the pathogen) that would significantly minimize the pathogen. There is simply too much evidence indicating that an environmental pathogen would be a hazard requiring a preventive control when these three conditions are met, even in some cases where the food is an RTE food that does not support the growth of environmental pathogens but has nevertheless been found to be contaminated (e.g., ice cream contaminated with Lm).
The costs associated with environmental monitoring can easily surpass the $10k mark in a single year, even in a small facility. Below are two examples of environmental monitoring provided in FDA’s Draft Guidance “Control of Listeria monocytogenes in Ready-To-Eat Foods,” published in January 2017: (1) for a RTE food capable of supporting Listeria (e.g., soft cheese) and (2) a food that is not capable of supporting Listeria but which has a history of contamination with Lm (e.g., ice cream). To calculate cost estimates, I will use FDA’s example frequencies and the Food Safety Guides Pathogen Environmental Monitoring (PEM) Calculator. The PEM Calculator is free for download on our website.
Example from the FDA Listeria Guidance
An example of how to specify the frequency of sample collection in a written environmental monitoring plan for food contact surfaces in an establishment producing an RTE food that supports growth of L. monocytogenes is as follows:
• Collect environmental samples from specific food contact surfaces on the production lines at least once every week when the plant is in operation; and
• Test each food contact surface in the plant at least once each month.
An example of how to specify the frequency of sample collection in a written environmental monitoring plan for non-food contact surfaces in an establishment producing an RTE food that supports growth of L. monocytogenes is as follows:
• Collect environmental samples from representative sets of non-food contact surfaces at least once weekly for zone 2 sites, every two weeks for zone 3 sites, and monthly for zone 4 sites when the plant is in operation; and
• Test all non-food contact surface sites identified in the monitoring plan at least once each quarter.
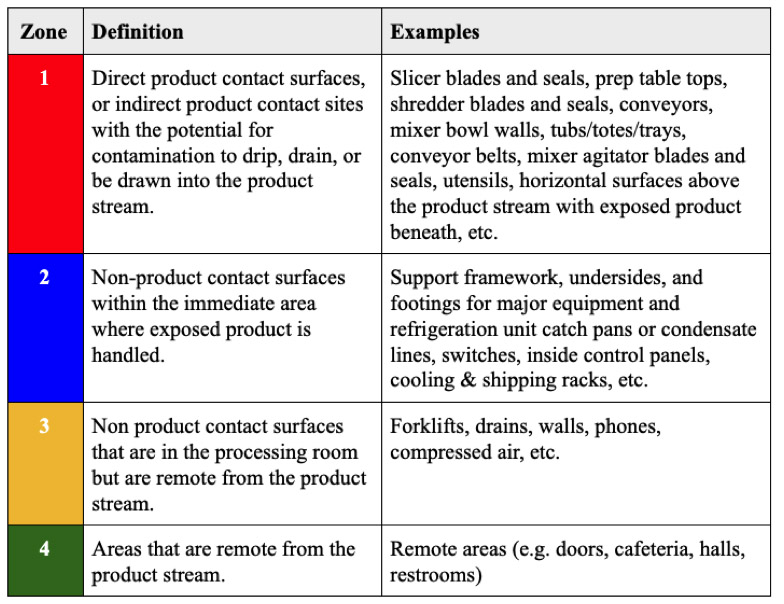
Breaking Surfaces Out into Zones
In order to determine which surfaces are swabbed at the frequencies described above, we will need to break up the surfaces into “zones.” Food contact surfaces are generally regarded as “zone 1” surfaces. Non-food contact surfaces are typically broken up into zones 2–4 as follows:
 Master Swab List
Master Swab List
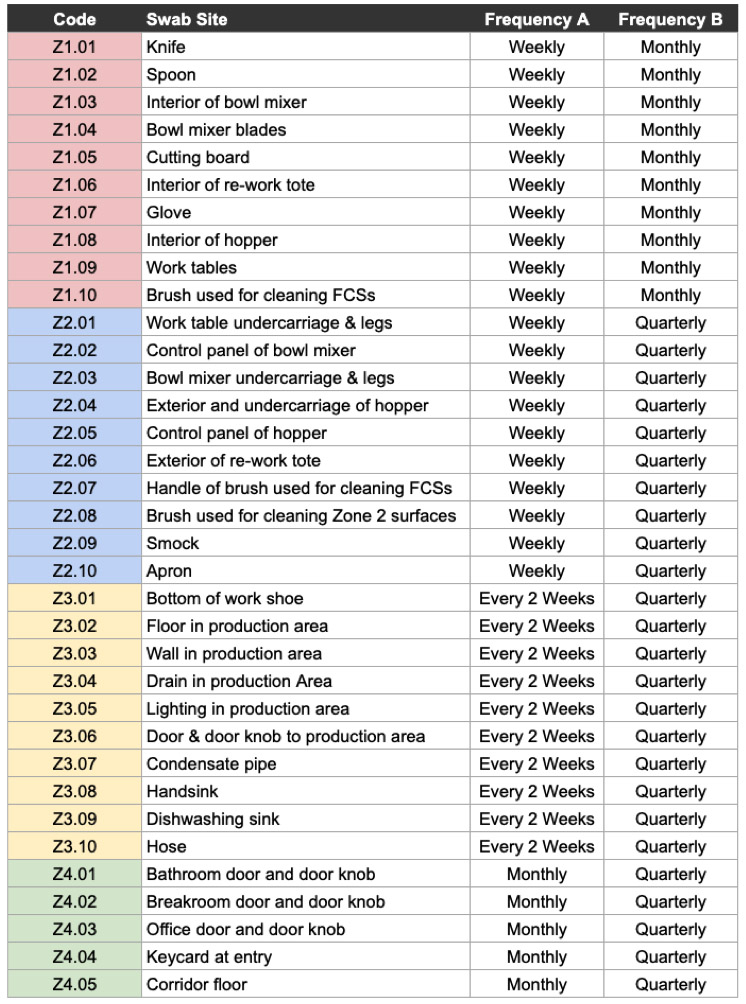
For the sake of simplicity, let’s assume in this example that we have:
(10×) Zone 1 surfaces
(10x) Zone 2 surfaces
(10x) Zone 3 surfaces
(5x) Zone 4 surfaces
I have listed surfaces in the table below. Swab frequency A represents the more rigorous minimum sampling frequency provided by FDA, which applies to RTE foods that support Lm; frequency B represents the less rigorous minimum sampling frequency, which applies to RTE foods that do not support Lm.
 Sample Master Swab List
Sample Master Swab List
Assumptions
To further simplify matters, I make the following assumptions in this example:
We are only testing for Listeria spp., an indicator organism that may indicate presence of Lm.
Each swab for Listeria spp. costs $10 (labs may charge more or less).
No compositing.[1]
No presumptive positives.[2]
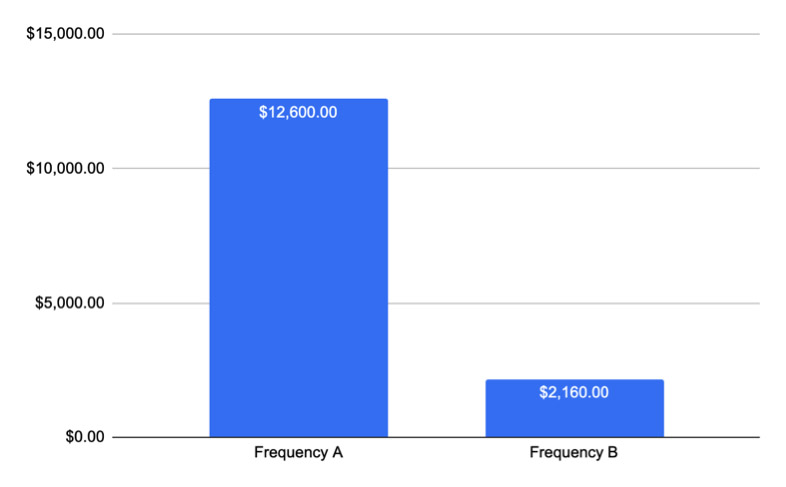
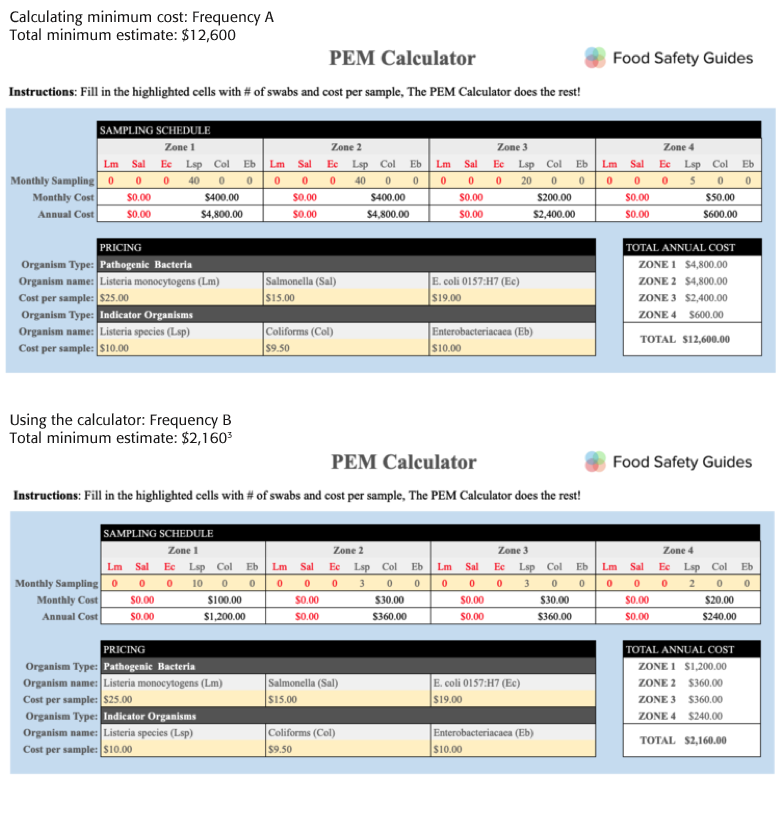
Estimated Costs
 Compositing refers to using a single swab to collect samples from multiple swab sites. Compositing can be very useful in that it saves money and can allow you to cast a much wider net with little extra cost, but if not done strategically, it can open a Pandora’s box of problems (e.g., you would never want to composite zone 1 surfaces because a positive would implicate every surface in that composite and create unnecessary liability, expense and anxiety). Similarly, you would never want to composite across zones (e.g., zone 2 and zone 3 surfaces). In the short-term, compositing may drive cost down by reducing the number of swabs, however if you get a hit (aka a “presumptive positive”), costs will go up significantly with the ensuring investigational swabbing. A single presumptive positive will result in a fusillade of swabs (strategic, of course) aimed at locating the pathogen (wherever it may be) and identifying the source and pathway of the pathogen (in the event the source is different from where the positive hit occurred), in addition to other activities (e.g., determining if other surfaces/foods could have been contaminated). How many swabs (and where) will be determined by the specific circumstances of the positive and what methodology the quality assurance team uses for their response. Approaches are described in a number of publicly available guidance documents.
Compositing refers to using a single swab to collect samples from multiple swab sites. Compositing can be very useful in that it saves money and can allow you to cast a much wider net with little extra cost, but if not done strategically, it can open a Pandora’s box of problems (e.g., you would never want to composite zone 1 surfaces because a positive would implicate every surface in that composite and create unnecessary liability, expense and anxiety). Similarly, you would never want to composite across zones (e.g., zone 2 and zone 3 surfaces). In the short-term, compositing may drive cost down by reducing the number of swabs, however if you get a hit (aka a “presumptive positive”), costs will go up significantly with the ensuring investigational swabbing. A single presumptive positive will result in a fusillade of swabs (strategic, of course) aimed at locating the pathogen (wherever it may be) and identifying the source and pathway of the pathogen (in the event the source is different from where the positive hit occurred), in addition to other activities (e.g., determining if other surfaces/foods could have been contaminated). How many swabs (and where) will be determined by the specific circumstances of the positive and what methodology the quality assurance team uses for their response. Approaches are described in a number of publicly available guidance documents.
Example: A Facility Gets a Presumptive Positive
Half way through the year, a manufacturer sampling according to Frequency A (e.g., soft cheese) gets a hit on a zone 2 surface during routine sampling and does the following (in addition to other measures):
• Collects 15 samples of adjacent and nearby zone 1, 2 and 3 surfaces. No composite swabbing. Samples are tested for Lm (not Listeria spp.).
• Swabbing of 15 sites is repeated for a total of three consecutive production shifts, all resulting in negative results.
Of the 15 sites sampled in the investigation, sampling frequency is increased for 1 month as follows (no compositing):
• Zone 1 sampling is increased from weekly to twice a week (5× surfaces)
• Zone 2 sampling is increased from weekly to twice a week (5× surfaces) for one month.
• Zone 3 surface sampling is increased from every two weeks to weekly (5× surfaces) for 1 month.
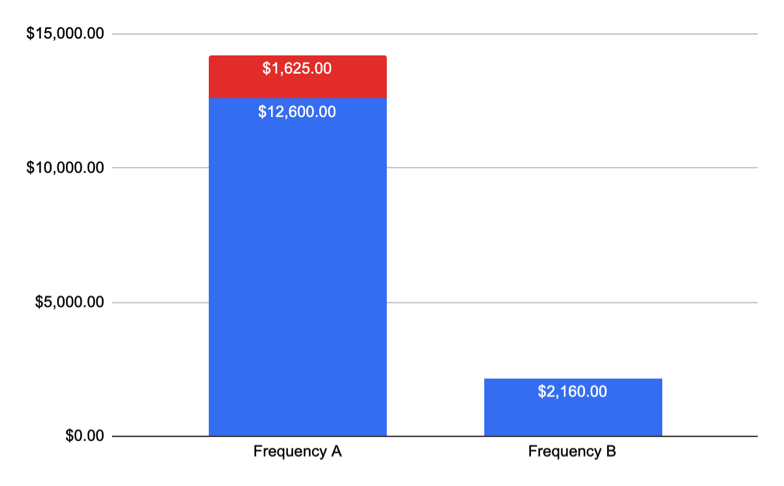 As a result, the supplier pays:
As a result, the supplier pays:
$1,125 for 45 investigational vector samples × $25/sample for Lm testing
$500 for 50 additional swabs for 1 month × $10/sample for Listeria spp. testing
Total additional cost: $1,625
Of course, the true cost would be more as investigational swabbing could be more aggressive and would/could include:
• Shipping and materials associated with increased swabbing
• Paying a consultant to advise on how to respond to incident
• Overtime paid while investigating issue and communicating with customers
• Holding/destruction of product
• Disruption to production schedule (opportunity costs)
If more positives were discovered [e.g., recurrence on same site(s) throughout the year or during the investigation] or positive was from a composite swab, cost would increase. If a positive was discovered on a zone 1 surface, costs associated with the recall and destruction of product would apply if affected product had been released into commerce (assuming product is not re-worked).
In this article, I could have gone much more in depth and played out scenarios with greater complexity, but the purpose of the article is to give a simple, straightforward example of what one might pay at a minimum.
For many facilities, it is unrealistic to assume that you will never encounter a presumptive positive. For this reason, I recommend adjusting calculations for multiple positives. Doing so will help ensure that you have an adequate budget as well as create realistic expectations. I also recommend calculating the costs associated with developing, implementing and maintaining related and supporting programs, for example (this is not intended to be exhaustive):
GMPs Facility maintenance Recall plan
Sanitation Procurement of equipment Traceability
Facility design Equipment maintenance Training
It pays (literally) to have a well-informed strategy when conducting environmental monitoring. By compositing smartly, you can save money; by searching diligently for pathogens in your environment, you strengthen your sanitation program; and, in the long term, you save money by preventing expensive recalls and damage to your brand. Most importantly, it gives you confidence (hopefully) that you are doing everything in your power to make safe food.
Charlie Kalish is a managing member of Food Safety Guides.
DISCLAIMER: The examples provided in this article are examples selected for illustrative purposes only. They are not intended to be used as a model or basis for a facility’s PEM program. Surfaces listed may not be representative of the type or number of surfaces appropriate for your facility. Every PEM program must address the specific risks posed by the facility, equipment/surfaces, processes, and food. Selection of indicator organisms/pathogens, swabs site placement, and swab frequency should be determined by a validated risk assessment performed by a qualified professional. Testing of swabs should be performed by a suitable laboratory, ideally one that is ISO 17025 accredited. To learn more about selecting a laboratory, check out my article in Food Safety Magazine here. If you have additional questions, you can reach me at info@foodsafetyguides.com.
References
1. Compositing refers to using one swab to collect samples from multiple surfaces. More information is provided on compositing below.
2. Realistically, we should expect to find presumptive positives. However, to set a baseline estimate I am assuming only negative results. If a positive result was detected, aggressive investigational swabbing would result, increasing the total estimate significantly.
3. Decimal values were rounded up in calculating monthly and quarterly swabs for zones 2–4.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →






