Biofilms and Microorganisms on Surfaces After Cleaning and Disinfection
It is common practice among food microbiologists to use the term “biofilm” to designate a community of microorganisms immobilized on a substratum as did Charaklis in 1989.[1] However, it should be noted that the word “biofilm” is currently used to mean “matrix-enclosed microbial populations adherent to each other and/or to surfaces or interfaces.”[2]
According to this definition, biofilms result from the growth of attached microorganisms which, due to the production of extracellular polysaccharides, are able to form micro- (Figure 1)[3] or macro-colonies on surfaces. In food processing plants, surfaces are periodically cleaned and disinfected, the temperature is generally low or the atmosphere is dry. Thus, the process of biofilm formation is periodically interrupted, and such conditions usually hamper the formation of microcolonies.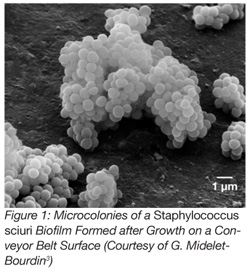
Furthermore, not all species are able to produce microcolonies or alternatively, the nutritive conditions do not always permit microcolony formation, which means that the term “biofilm” is not necessarily correct for referring to all bacterial communities attached to surfaces in food processing plants. Phenotypic resistance to disinfectants at bactericidal concentrations occurs among all attached cells whether they are in a biofilm state or not. Consequently, the following discussion will focus on microorganisms found on surfaces after cleaning and disinfection (C&D), which is the main concern for food processors.
Heterogeneity of Microbial Populations
When a bacterial suspension comes into contact with a surface, only some of the cells are able to adhere. For instance, after deposition of a bacterial suspension of Listeria monocytogenes on a glass slide, the number of attached cells stops increasing after 2.5 hours and then accounts for only 1% of the suspended cells.[4] When an attached community of bacterial cells is subjected to C&D, only a few are able to resist. This fraction of surviving cells is smaller when disinfectant concentration increases and is greater for old attached communities. Such fractions also exist in bacterial suspensions but are much smaller, which is why attached cells are considered to be more resistant than suspended cells. This minority subpopulation consists of cells that are referred to as “persister cells.”[5] Persister cells are phenotypic variants that appear frequently but in an unpredictable manner, resulting in a great variability between the number of cells surviving C&D. Consequently, there must be a sufficiently high number of initially attached cells for there to be any chance of finding “persister cells” after C&D when applied at the recommended concentrations.[6] A few cells may survive even when their initial number is not that high. Of course, the initial number of cells necessary to ensure the certainty of some cells surviving varies according to the bacterial species and strains and depends on the attachment strength and intrinsic resistance to C&D of the microbial strain considered.
Cleaning and Disinfection
Surfaces are cleaned to remove soiling and certain microorganisms and then disinfected to inactivate any microorganisms that were not eliminated by the cleaning. The objective is to reduce microbial counts so that the microbiological quality of the product is satisfactory. On surfaces in food processing plants, some bacterial cells always survive C&D. When assessing C&D efficacy, two points must be taken into account to avoid an erroneous interpretation. First, swabbing methods used to sample surfaces do not detach all the microbial cells and the detached fraction is lower after C&D. The second concern is that although some cells are not culturable, they may still be alive. We recently observed that incubating plates for 6 days instead of 3 days at 25 °C led to greater colony-forming unit (CFU) counts from samples taken after C&D in a meat site; incubating plates for 14 days at the same temperature further increased CFU counts. In the same study, using ethidium monoazide real-time polymerase chain reaction (EMA-qPCR), the numbers of viable cells from food contact surfaces were shown to be significantly greater than CFU numbers on stainless steel equipment after C&D. One to 10 CFU/cm{2} were detected on agar medium, while viable cells detected by EMA-qPCR ranged from 10{3} to 10{4} cells/cm{2}, and total cells detected by qPCR ranged from 10{3} to 10{5} cells/cm{2}.[7]
Bacterial genera found after cleaning and disinfection. When comparing studies conducted in factories processing food of animal origin, Pseudomonas and Staphylococcus are usually dominant. However, the development of culture-independent methods for bacterial identification might lead to another view of ecosystems,[8] where Sphingomonadaceae were dominant on a conveyor belt in a lamb processing plant. This was done using a culture-independent technique, whereas by culture, Pseudomonas was found to be in the majority.
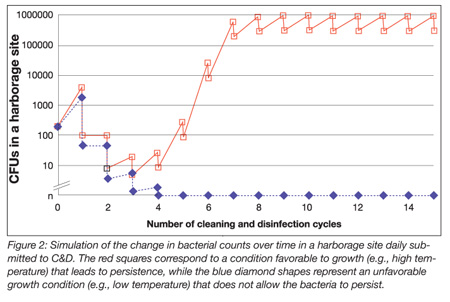
Bacterial persistence. Among bacterial species found on surfaces after C&D, some were shown by means of molecular subtyping methods to be persistent. Among those bacteria, the most well known are L. monocytogenes in refrigerated, wet processing plants and Salmonella enterica in dry ones. However, persistence should not be confused with transient survival or permanent entrance of a strain in unprocessed food or ingredients. A recent review investigating the persistence of L. monocytogenes[9] concluded that it was highly probable that there were no L. monocytogenes strains with unique properties leading to persistence, but rather harborage sites in which L. monocytogenes was able to survive C&D, multiply and resist recommended concentrations of disinfectants. When growth exceeds destruction by C&D, microorganisms inevitably become persistent. Consequently, as shown in Figure 2, the best way to prevent persistence is to hamper bacterial growth.
Recommendations
The most important recommendation is that equipment and premises should be hygienically designed so that bacteria cannot find harborage sites that protect them from cleaning. European Hygienic Engineering & Design Group documents should be consulted in this respect. Then, maintaining a low ambient temperature and dryness are powerful ways to decrease bacterial growth. Intermittent dryness can be achieved by taking the following preventive measures: slopes of floors and equipment must allow drainage; drains and U-bends must be correctly placed and in sufficient numbers; footbaths should be avoided; cold spots where water condenses should be removed; and, finally, a dehumidification system should be installed and used after C&D to allow surfaces to dry when the factory is not busy. Dryness, however, does not prevent bacteria from surviving. Gram-positive bacteria such as L. monocytogenes and Enterobacteriaceae such as Salmonella are able to endure hydric stress. In the event an undesirable strain persists in a processing plant, it is possible to avoid it circulating, including within aerosols. For instance, aerosols may form from drains when the flow rate suddenly increases. As L. monocytogenes can be present in such aerosols, covering drains will prevent circulation of the pathogenic bacterium. The inner surfaces of hoses may be colonized by spoilage bacteria, such as Pseudomonas, following airborne contamination of the end of water pipes.[10] To prevent such colonization, hoses should not be left lying on the floor, and the ends of the water pipes should be immersed in a disinfecting solution between successive uses. Finally, floors on which L. monocytogenes is frequently found are often made of unhygienic materials[11] as seen in Figure 3. Floors must be cleaned prior to equipment C&D so that microorganisms transferred from floors to food contact surfaces are eliminated.
.jpg)
Conclusions
Twenty years ago, widespread interest in biofilm contamination led many scientists to study biofilm formation, by comparing strains or culture conditions or looking for genetic pathways involved in biofilm formation. Unfortunately, few studies have integrated all the events and phenomena leading to the survival of microbial cells after C&D under conditions that are representative of those encountered in food processing plants. Such an approach is not easy but should be followed to gain more understanding of what is actually happening on surfaces in food processing plants.
Brigitte Carpentier, Ph.D. is in charge of the Research Team “Microbial Ecophysiology and Hygiene of Surfaces” at Maisons-Alfort Laboratory for Food Safety, French Agency for Food, Environmental and Occupational Health & Safety. She received a Ph.D. in Algology from the University of Paris VI.
References
1. Characklis, W. G. 1989. Microbial biofouling control. In W. G. Characklis and K. C. Marshall (Eds.), Biofilms (Vol. 15, pp. 585–631). New York: Wiley.
2. Costerton, J., Z. Lewandowski, E. Douglas, and R. Korber. 1995. Microbial biofilms. Annu Rev Microbiol 49:711–745.
3. Midelet, G. 2002. Etude des transferts microbiens par contact depuis des surfaces inertes vers un aliment. Application à la situation industrielle des bandes convoyeuses utilisées dans l’industrie de la viande. Ph.D. Thèse d’Université; Université de Bourgogne.
4. Sommer, P. 1999. Modification des équilibres microbiens au sein de biofilms colonisant les ateliers industriels fromagers. Thèse d’Université; Université de Bourgogne.
5. Lewis, K. 2005. Persister cells and the riddle of biofilm survival. Biochem (Moscow) 70:267–274.
6. Marouani-Gadri, N., O. Firmesse, D. Chassaing, D. Dandris-Nielsen, N. Arenborg and B. Carpentier. 2010. Potential of Escherichia coli O157:H7 to persist and form viable but non-culturable cells on a food-contact surface subjected to cycles of soiling and chemical treatment. Int J Food Microbiol 144:96–103.
7. Khamisse, E. O. Firmesse, D. Chassaing, S. Christieans and B. Carpentier. 2010. Quantification of the bacterial load of food contact surfaces from a meat processing plant before and after cleaning and disinfection. Presented at the 22nd International Committee on Food Microbiology and Hygiene Symposium Food Micro, Copenhagen.
8. Brightwell, G., J. Boerema, J. Mills, E. Mowat and D. Pulford. 2006. Identifying the bacterial community on the surface of Intralox belting in a meat boning room by culture-dependent and culture-independent 16S rDNA sequence analysis. Int J Food Microbiol 109:47–53.
9. Carpentier, B. and O. Cerf. 2011. Review – Persistence of Listeria monocytogenes in food industry equipment and premises. Int J food Microbiol 145:1–8.
10. Gagnière, S., F. Auvray and B. Carpentier. 2006. Spread of a green fluorescent protein-tagged Pseudomonas putida in a water pipe following airborne contamination. J Food Prot 69:2692–2696.
11. Carpentier, B. 2005. Improving the design of floors. In H. L. M. Lelieveld, M. A. Mostert and J. T. Holah (Eds.) Handbook of hygiene control in the food industry (pp. 168–182). Cambridge: Woodhead Publishing Ltd. >
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →







