Cultivating Cannabis Regulations: Ensuring Food Safety in an Evolving Industry
With the proliferation of cannabis legislation in many U.S. states, there is a need to address food safety oversight through a federal regulatory framework

Food trends are in a constant state of flux, shaped by changing consumer preferences for organic, vegan, and plant-based options, as well as considerations for dietary restrictions like those for people with diabetes and people adhering to religious dietary requirements. These trends also encompass concerns related to ingredient sourcing and animal welfare. They include the introduction of novel ingredients like yuzu, alternative proteins, and even insects into food products in the U.S.
The cannabis sector is a hotbed of innovation, especially when it comes to food, beverages, and dietary supplements. In states where cannabis is legalized, consumers have access to a wide variety of cannabis-infused food products, including candies, soft drinks, and capsules. Certain markets even offer convenient packs of cannabis-based ingredients to enhance culinary creations and beverages in the home kitchen.
These ever-changing food trends, combined with the absence of federal regulatory oversight for cannabis, have given rise to significant risks to public health and safety. The core problem lies in the fact that cannabis and cannabis-derived substances are not considered legal food additives by the U.S. Food and Drug Administration (FDA). When introducing new food additives not already classified as Generally Recognized as Safe (GRAS), organizations have the option to petition FDA for approval. This procedure entails a comprehensive safety assessment, covering aspects such as identification, potential exposure, safety evaluation, and usage restrictions.
In November 2022, FDA took action by issuing Warning Letters to four companies, underlining that cannabidiol (CBD) lacks approval as a food additive and these companies were in violation of the Food, Drug, and Cosmetic Act (FD&C).1 FDA continues to express concerns regarding the prolonged use of CBD and its appeal to children. As recently as July 2023, FDA warned six companies for illegally selling copycat food products containing Delta-8 THC.2 Previously, FDA had warned about the serious health risks of consuming Delta-8 THC after receiving over 100 reports of adverse events from January 1, 2021 through May 31, 2022.3
With the proliferation of cannabis legislation in the majority of U.S. states, there has been a growing number of incidents involving cannabis-infused foods and concerns about formal food safety oversight. The regulation of these edibles remains a source of industry confusion, with many consumers unaware of the limited oversight from a conventional food safety perspective when it comes to purchasing and consuming such products. There is a common assumption among consumers that if a product is legally available, it must be subject to government regulation. However, due to the complex legal landscape surrounding these cannabis products, the federal government does not regulate them, and state-level regulations vary drastically, often leading to inefficiencies and inconsistent standards that jeopardize public health and safety. This emerging product category is evolving rapidly, outpacing the ability of regulators and food safety experts to ensure that appropriate measures are in place to safeguard public health and safety.
History of Cannabis Regulation in U.S.
A brief summary of the history of Cannabis regulation in the U.S. outlines its evolution. In the early 20th century, Cannabis was federally legal, but shifting public opinions led to state-level restrictions. A pivotal moment came in 1937 with the Marihuana Tax Act,4 which differentiated "hemp" from other Cannabis varieties, allowing more lenient regulations on industrial hemp. However, the act was deemed unconstitutional in 1969, leading to the Controlled Substances Act (CSA) of 1970.5 This CSA categorized Cannabis as a Schedule I drug, signifying it had no medical value. Hemp shared this classification until the 2018 Farm Bill,6 which excluded it from the CSA's "marijuana" definition, permitting legal cultivation. Simultaneously, states like California took individual actions, notably legalizing medical cannabis in 1996 and then later adult use,7 creating a complex interplay between state and federal regulations that endures today.
Cannabis regulation in the U.S. is undergoing constant changes. While more states are legalizing its use, the federal government maintains a consistent stance, resulting in a complex and contradictory web of state-level regulations. The absence of a unified regulatory structure poses significant challenges, particularly concerning public health and safety. The issue can become more intricate when state regulators lack adequate training in food safety and approach inspections from an agricultural rather than a consumable human food product perspective. This can result in significant oversights in food safety standards, such as allergen management, adequate hygienic zoning, pathogen testing, ready-to-eat (RTE) handling, appropriate analytical and microbiological testing, and others, thereby jeopardizing consumer health and safety. This is further complicated by the fact that state regulators typically are not appropriately educated or adequately trained on the processes cannabis undergoes during production and manufacturing. This leads to increased health and safety gaps as state inspections often overlook major and critical deficiencies, due to the lack of understanding of the process.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
This article explores the efforts of the Federal Regulatory Framework (FRF) Working Group, a collaboration between the Foundation of Cannabis Unified Standards (FOCUS) and the Association of Food and Drug Officials (AFDO). The Working Group's goal is to develop a comprehensive and relevant roadmap for the regulatory framework of these unique consumer packaged goods (CPG), addressing critical challenges and enhancing consumer confidence in the safety of the products they purchase and consume. The complexity within the food and beverage industry stems from cannabis not having approval as a food additive, and as a result, cannabis companies are producing new consumer products categorized as food without comprehensive regulation or oversight by food safety experts and regulatory bodies.
Cannabis Food Safety Regulatory Landscape
Federal jurisdiction over hemp and cannabis is divided among three primary agencies: the U.S. Department of Agriculture (USDA), FDA within the Department of Health and Human Services (HHS), and the Drug Enforcement Administration (DEA) within the Department of Justice (DOJ). USDA governs hemp cultivation and related agricultural programs, while FDA regulates food and food products, including those containing CBD and other cannabinoids derived from hemp, all of which currently lack FDA approval.8 DEA maintains control over Cannabis as a Schedule I drug,9 making non-hemp Cannabis illegal at the federal level.
On the state level, 37 states and three U.S. territories, along with the District of Columbia, have legalized medical cannabis, while 21 states, Guam, and the District of Columbia have legalized adult-use cannabis.10 States employ different regulatory models, leading to uncertainty and complexity for cannabis businesses. The interplay between state and federal laws remains unclear due to the Supremacy Clause, creating confusion and inconsistent outcomes for the public. The Supremacy Clause in the U.S. Constitution asserts that the Constitution itself, federal laws created in accordance with it, and treaties established under its jurisdiction are deemed the "highest Law of the Land," thus superseding any contradictory state laws. The main issue with the current state-level oversight is that states do not regulate the process, but rather test the finished products.
The federal approach to cannabis enforcement remains uncertain and variable. In 2013, during President Obama's administration, Deputy Attorney General James M. Cole issued the Cole Memorandum,11 which provided guidance to U.S. attorneys not to oppose state laws legalizing cannabis. Nevertheless, this policy was later reversed during the Trump Administration12 and then reinstated during the Biden Administration,13 leading to ongoing industry and consumer confusion.
The confusion extends to food and beverage products containing cannabis-derived ingredients, which would traditionally fall under FDA's jurisdiction. While FDA recognizes the potential benefits of cannabis, it struggles to regulate these products due to violations of the FD&C Act, as cannabis is not considered a legal food additive.14 FDA primarily relies on an enforcement-based approach, issuing warning letters to companies that violate regulations. However, this approach has not kept pace with the market's growth, leaving a lack of oversight for cannabis companies due to their inherent federal law violations.
Restrictions on the interstate transportation of cannabis result in a fragmented market, where legal cannabis is limited to certain states. This compels businesses to set up multiple facilities and often adopt a vertical supply chain, leading to economic inefficiency. This not only burdens businesses and vendors but also impacts medical cannabis patients who cannot carry their prescribed treatments across state lines, even between states with legal medical cannabis. These constraints on interstate movement hinder industry coherence and efficiency, affecting consumer safety and patient well-being, making it essential to address these challenges in the current cannabis regulatory structure.
An urgent need for a comprehensive federal regulatory framework for cannabis is evident. The existing mosaic of state regulations presents a significant risk to public health and safety. Unfortunately, the federal ban on Cannabis limits FDA's traditional role in ensuring public safety and standards, resulting in uneven and often ineffective oversight, particularly for food and beverage items and dietary supplements such as edibles, soft drinks, capsules, and various supplements targeting issues like sleep, pain, and anxiety.
What is notable in this regulatory landscape is the disproportionate emphasis on physical security measures at the expense of consumer health and safety, driven by the enduring stigma associated with Cannabis as an illegal drug. The Federal Regulatory Framework Working Group is steadfast in its mission to prioritize safe and wholesome consumption, without taking a stance on the moral debate surrounding cannabis legality and use. Comprising experts from diverse industries, the group shares a common belief: that all legal cannabis products should uphold consumer health and safety, leaving no room for harm due to insufficient oversight.
Food Safety and Consumer Understanding in the Cannabis Industry
Food safety becomes intricate in the absence of defined production standards and risk-based, scientifically grounded testing. This deficiency jeopardizes consumer health and safety. Several issues arise due to the current industry's lack of standards, including inconsistencies in laboratory practices, processes, verifications, validations, and essential protocols necessary for reliable laboratory data. Variability and lack of appropriate oversight also exist in laboratory procedures, highlighting the significance of Good Laboratory Practices (GLPs) and the insufficiency of in-house laboratories without proper controls, which yield dissimilar data compared to accredited third-party laboratories. This leads to situations where favorable results from one retailer may lack data integrity, while another retailer employing accredited laboratories maintains rigorous procedures and data integrity.
The consequences of utilizing in-house laboratories versus accredited third-party laboratories result in dramatically divergent testing outcomes. Just because one laboratory deems a product safe or assesses it for microorganisms with favorable results does not necessarily imply accuracy or validity. The lack of cohesive standards presents issues with the relevance of the parameters being tested. (i.e., lacking science-based foundation). Mycotoxins and pesticide residues also pose risks to consumer safety, demanding the appropriate testing and sampling methods, as well as laboratory accreditation. Additional food safety concerns encompass cannabis industry nuances, such as irradiation and remediation, that yield favorable test outcomes but may not be inherently wholesome or transparent to consumers due to a lack of labeling regulations. Moreover, the use of terms like "organic" and "natural" in the context of cannabis does not consistently align with their definitions in other industries, potentially misleading consumers that have become accustomed to traditional food industry standards.
Consumer comprehension is further complicated by implicit rather than explicit claims about cannabis. While FDA governs health claims on products, in the cannabis market, companies may not directly make these claims, but consumers often infer them from various information sources. For example, research suggests that CBD may offer potential benefits in alleviating pain, stress, and sleep issues. Consequently, consumers may seek out these products and assume they are suitable for these purposes.
Due to the lack of comprehensive regulations in the production of CBD-infused items, not all products are manufactured with adequate safety measures. For example, in 2020, a recall in Florida identified elevated lead levels in a batch of CBD tinctures.15 Other cannabis products may carry risks such as Aspergillus, pathogenic E. coli, and Salmonella. In June 2023, an Arizona cannabis company recalled certain products due to possible Aspergillus and Salmonella contamination, which was a result of ADHS auditors discovering falsified records that were subsequently reported by the licensed cannabis laboratory.16 This presents a challenge, as consumers frequently believe they are purchasing safe products held to the same standards as traditional CPG products in states with legalized cannabis. The risk to consumers is amplified when these products are marketed to specific demographics, such as the elderly or individuals with certain medical conditions, who are more vulnerable to adverse effects when exposed to such pathogens.
Cannabis Regulatory Partnership: AFDO and FOCUS
In response to this continually evolving and intricate challenge, AFDO and FOCUS united their efforts. The two groups have been in collaboration since 2017 through a Cannabis Regulatory Partnership, with the aim of presenting a solution to the regulatory inconsistencies that put the public at risk. This partnership led to the establishment of the Federal Regulatory Framework (FRF) Working Group.
AFDO and FOCUS are two pivotal organizations renowned for their commitment to public health and safety. AFDO, with a rich history spanning over a century, specializes in food, drugs, and the manufacturing of downstream products.17 Conversely, FOCUS is dedicated to quality, health, and safety within the cannabis and cannabis products sector.18 The AFDO/FOCUS partnership serves as a crucial link between all industries intersecting with cannabis, uniquely positioning itself to offer an impartial, comprehensive solution for effective and all-encompassing federal cannabis regulation.
Federal Regulatory Framework (FRF) Working Group
In June 2022, the collaborative FRF Working Group convened in Indianapolis, Indiana to initiate the development of a regulatory framework for the industry. This meeting served as the foundation for the FRF Working Group's efforts, marking the commencement of discussions regarding the creation of a whitepaper and the formulation of a strategy for advocating and presenting research to the relevant stakeholders.
The group consists of distinguished professionals from various fields of expertise. Recruited by the founder of FOCUS, Lezli Engelking, I was selected for my extensive experience in the field of food safety and regulatory science, having worked in this regulated industry for over 15 years. I was also selected for my overt passion for activism on topics concerning public health and safety. Other members include a forensic toxicologist, cannabis business proprietors, chemists, scientists, former regulators, attorneys, political experts, and individuals with diverse experiences crucial to comprehending and resolving this intricate issue. Each member makes a significant and industry-leading contribution to the regulatory framework being formulated for cannabis-infused products. A full list of members and their short bios can be found on the Federal Regulatory Framework website.19
FRF Working Group Mission
The Federal Regulatory Framework Workgroup was established to guide Congress and U.S. federal regulatory agencies in developing a federal cannabis regulation approach. This approach prioritizes public health and safety, relies on scientific practices, accommodates the diverse uses of the Cannabis plant, integrates best practices from established industries, garners broad support, enhances the U.S. position in the global cannabis market, and encourages industry participation from all stakeholders.
The Workgroup aims to:
- Create an impartial forum focused on public health and safety in U.S. cannabis regulation
- Engage a wide range of stakeholders, leveraging their operational knowledge and experience
- Establish a sustainable model emphasizing technical excellence, comprehensiveness, durability, and suitability
- Foster broad consensus among numerous stakeholders
- Educate on the current public health and safety risks stemming from federal non-involvement
- Allow for state-specific regulations
- Consider the downstream effects of both state and federal legalization, including impairment and public safety risks.19
FRF Working Group Model
The outcome of our collective endeavors, originating from that pivotal gathering in Indianapolis, resulted in the creation of a comprehensive regulatory framework model illustrated in Figure 1. This framework is designed to address the existing gaps and disparities within the current regulatory landscape, with a steadfast commitment to ensuring the safety and quality of cannabis products. The group discerned and categorized key product types for cannabis products, as illustrated in Figure 1. Each product category aligns with an existing regulatory authority and framework. This model includes the existing framework, with the exception of the need for a new cannabinoid regulatory agency.
A meticulous assessment of cannabis products, their applications, and their impact on consumers revealed that attempting to place all cannabis products under a single agency, such as FDA, would be inappropriate and grossly insufficient. The FRF Working Group identified four core categories encompassing industrial, food and ingredients, therapeutic/psychotropic, and pharmaceutical pathways. The degree of regulation was determined by factors like product examples, THC levels, consumer access channels, monitoring mechanisms, and the respective regulatory body. These conclusions and assignments were comprehensively detailed in the FRF Working Group's whitepaper.
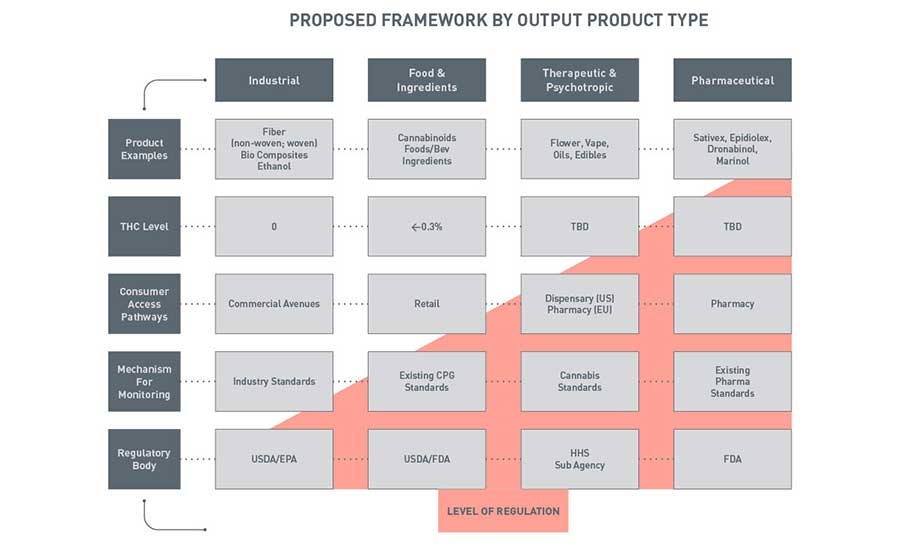
The whitepaper, a culmination of the FRF Working Group members' collective expertise, offers a blueprint for the future of cannabis regulation in the U.S. It outlines the dire need for stringent standards for cultivation, processing, packaging, and distribution of cannabis products, emphasizing the critical role of oversight in safeguarding public health and safety. The collaborative efforts of the FRF Working Group have borne fruit in the form of a robust, all-encompassing framework that holds the promise of a safer, more reliable cannabis industry for all product types including food and beverage.
FRF Working Group Whitepaper Public Review
The work is far from over. The next steps for the Federal Regulatory Framework Working Group involve garnering support and awareness for the comprehensive Regulatory Framework in two key phases. Phase 1 is designed as the starting point for refining the discussion around the proposed Framework and associated stakeholder groups in preparation for public comments. Organizations representing the various impacted stakeholder groups will be invited to help refine and improve the initial draft of the whitepaper developed by the FRF Workgroup. Feedback will be incorporated into the draft for Phase 2 Review. We plan to engage with key stakeholders, policymakers, and influencers to advance our mission.
Phase 2 will be a public review. In an effort to encourage broad participation by all interested stakeholders, the draft whitepaper will be opened up for public review and comment for 45 days. Additional details on how to participate will be added to the Federal Regulatory Framework website. To receive updates on this project, or to participate in the public review, interested parties can fill out the contact form on the website.
In conclusion, the Federal Regulatory Framework Working Group, a joint effort between FOCUS and AFDO, is poised to reshape the future of cannabis regulation in the U.S.20 Our vision is clear: to establish a comprehensive cannabis regulatory framework that prioritizes public health and safety above all else. As the cannabis industry continues to evolve, our commitment remains unwavering, and our mission remains resolute: to cultivate a safer, healthier future for all. Stay updated on our progress by visiting the FRF Working Group's website and exploring the resources available.
References
- U.S. Food and Drug Administration (FDA). "FDA Warns Companies for Illegally Selling Food and Beverage Products that Contain CBD." November 21, 2022. https://www.fda.gov/food/cfsan-constituent-updates/fda-warns-companies-illegally-selling-food-and-beverage-products-contain-cbd.
- FDA. "FDA, FTC Warn Six Companies for Illegally Selling Copycat Food Products Containing Delta-8 THC." July 6, 2023. https://www.fda.gov/news-events/press-announcements/fda-ftc-warn-six-companies-illegally-selling-copycat-food-products-containing-delta-8-thc.
- FDA. "5 Things to Know about Delta-8 Tetrahydrocannabinol—Delta-8 THC." May 4, 2022. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc.
- U.S. Customs and Border Protection. "Did You Know… Marijuana Was Once a Legal Cross-Border Import?" December 20, 2019. https://www.cbp.gov/about/history/did-you-know/marijuana.
- U.S. Drug Enforcement Administration (DEA). "The Controlled Substances Act." https://www.dea.gov/drug-information/csa.
- U.S. 115th Congress. H.R.2—Agriculture Improvement Act of 2018. December 20, 2018. https://www.congress.gov/bill/115th-congress/house-bill/2.
- Department of Cannabis Control California. "California's cannabis laws." 2023. https://cannabis.ca.gov/cannabis-laws/laws-and-regulations/.
- FDA. "FDA Concludes that Existing Regulatory Frameworks for Foods and Supplements are Not Appropriate for Cannabidiol, Will Work with Congress on a New Way Forward." January 26, 2023. https://www.fda.gov/news-events/press-announcements/fda-concludes-existing-regulatory-frameworks-foods-and-supplements-are-not-appropriate-cannabidiol.
- DEA. "Drug Fact Sheet: Marijuana/Cannabis." June 2020. https://www.dea.gov/sites/default/files/2020-06/Marijuana-Cannabis-2020_0.pdf.
- DISA Global Solutions. "Marijuana Legality by State." October 1, 2023. https://disa.com/marijuana-legality-by-state.
- U.S. Department of Justice. "Memorandum for All United States Attorneys: Guidance Regarding Marijuana Enforcement." August 29, 2013. https://www.justice.gov/iso/opa/resources/3052013829132756857467.pdf.
- U.S. Congress. "Letter to President Donald J. Trump." January 25, 2018. https://www.cantwell.senate.gov/imo/media/doc/01252018%20Letter%20to%20Trump%20on%20Sessions%27%20withdrawal%20of%20the%20Cole%20memo.pdf.
- U.S. White House. "Statement from President Biden on Marijuana Reform." October 6, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/10/06/statement-from-president-biden-on-marijuana-reform/.
- FDA. "FDA Regulation of Dietary Supplement & Conventional Food Products Containing Cannabis and Cannabis-Derived Compounds." https://www.fda.gov/media/131878/download.
- FDA. "Summitt Labs Issues Voluntary Nationwide Recall of KORE ORGANIC Watermelon CBD Oil Due to High Lead Results." July 28, 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/summitt-labs-issues-voluntary-nationwide-recall-kore-organic-watermelon-cbd-oil-due-high-lead.
- Arizona Department of Health Services. "Voluntary recall of certain marijuana products due to possible Aspergillus contamination." June 14, 2023. https://www.azdhs.gov/director/public-information-office/index.php#news-release-061423.
- Association of Food and Drug Officials (AFDO). "About AFDO." 2023. https://www.afdo.org/about/.
- Foundation of Cannabis Unified Standards (FOCUS). "Our Company." 2023. https://www.focusstandards.org/company-2/.
- Federal Regulatory Framework. "Workgroup Objectives." 2023. https://www.federalregulatoryframework.com/workgroup/.
- AFDO. "Public health and safety organizations engage workgroup to develop regulatory framework for cannabis in the U.S." June 28, 2022. https://www.afdo.org/wp-content/uploads/2022/06/AFDO.FOCUS-Framework-Media-Release-Final.pdf.
Cori Muse is a Food Safety and Regulatory Consultant and the Owner of Muse Food Safety Solutions LLC. She is also an independent GFSI Auditor, an alumni of PepsiCo/Frito-Lay, and has an extensive background working in the food industry over 14 years. She can be reached at solutions@foodsafetymuse.com.









