Developing Thermal Control of Salmonella in Low-Moisture Foods Using Predictive Models
Salmonella is highly resistant to heat inactivation in low-moisture food systems, but pilot-scale thermal treatments show promise for combatting it

Over the past decades, concerns over the safety of low-moisture foods have grown due to numerous outbreaks and recalls associated with various products. Among the involved pathogens, Salmonella spp. emerged as a prevalent concern.1 While thermal pasteurization has effectively reduced the risk of pathogen contamination in high-moisture foods, vegetative bacterial pathogens like Salmonella spp. have shown high thermal tolerance in low-moisture foods. The thermal processing conditions designed for high- and intermediate-moisture foods are inadequate for achieving sufficient inactivation of bacterial pathogens in low-moisture foods. Therefore, it is crucial to gain a comprehensive understanding of the key factors influencing the thermal resistance of bacterial pathogens, particularly Salmonella.
This article presents the latest research findings that shed some light on the reasons behind the enhanced thermal resistance of foodborne pathogens in low-moisture food systems. It discusses the quantitative relationships between thermal treatment temperature, water activity, and relative humidity and their impact on bacterial pathogens' log-reduction rate (D-value). Furthermore, it presents case studies that demonstrate the development and validation of predictable thermal treatments for Salmonella control in pre-packaged food materials, like wheat flour, and on the surfaces of food particles, such as peppercorns.
Thermal Tolerance of Bacterial Cells in Low-Moisture Environments
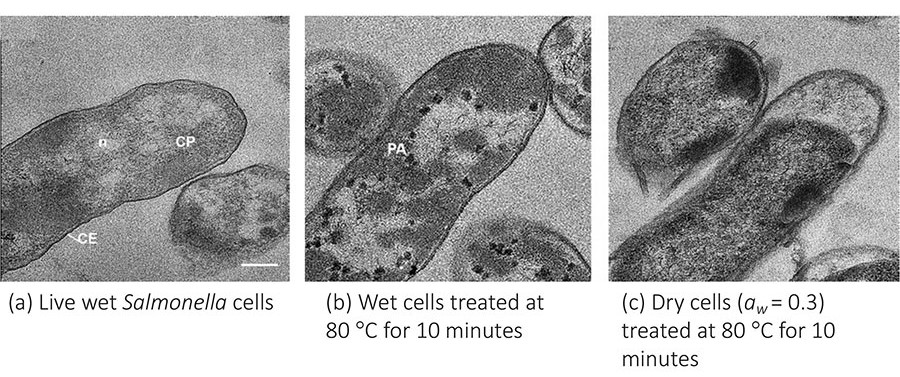
The thermal death of bacteria occurs through irreversible changes in critical cell components.2 Desiccated vegetative bacteria cells have demonstrated remarkably high thermal resistance, even comparable to that of spore-formers like Bacillus and Clostridium.3 While the role of water in the thermal death of microorganisms is not yet clearly understood, the absence of water likely restricts molecular mobility and protein deformation in living cells, thereby reducing the rate of denaturation of the cell's ultrastructure and prolonging the time required to cause irreparable damage.2,4,5 Figure 1 illustrates how desiccated Salmonella cells did not exhibit clear protein aggregations as did wet bacterial cells after undergoing the same thermal treatment (80 °C, 10 minutes).
Correlating Thermal Resistance Data with Measurable Parameters
The thermal death kinetics of bacteria are commonly modeled using a first-order reaction, which suggests that each bacterial cell has the same probability of dying under a specific treatment condition.6 Temperature was a key factor influencing the reaction rate, and its impact on the rate coefficient, in general, follows the Arrhenius equation.7,8 Early studies focused on sterilization or pasteurization of high- or intermediate-moisture foods and did not include the influence of food water activity. Research on pathogen control in low-moisture foods has significantly increased over the past two decades. It is now widely recognized that the thermal death of bacteria, such as Salmonella, Listeria, and E. coli, in low-moisture foods can also be effectively modeled using first-order kinetics.9 At any given temperature, however, the thermal inactivation rates for the bacteria in low-moisture foods were much lower than those in high moisture-content foods.10
Efforts have been made to establish connections between bacteria's thermal death time (D-value) and measurable environmental factors, including temperature and water activity (aw), to guide the design of thermal processes for low-moisture food commodities. In early studies, water activity was considered a temperature-independent factor when measuring the thermal resistance of bacteria. While this assumption may be true for high- or intermediate-moisture food matrices that contain free water, it does not apply to low-moisture foods that have multilayers of water molecules bonded with different levels of energy.11
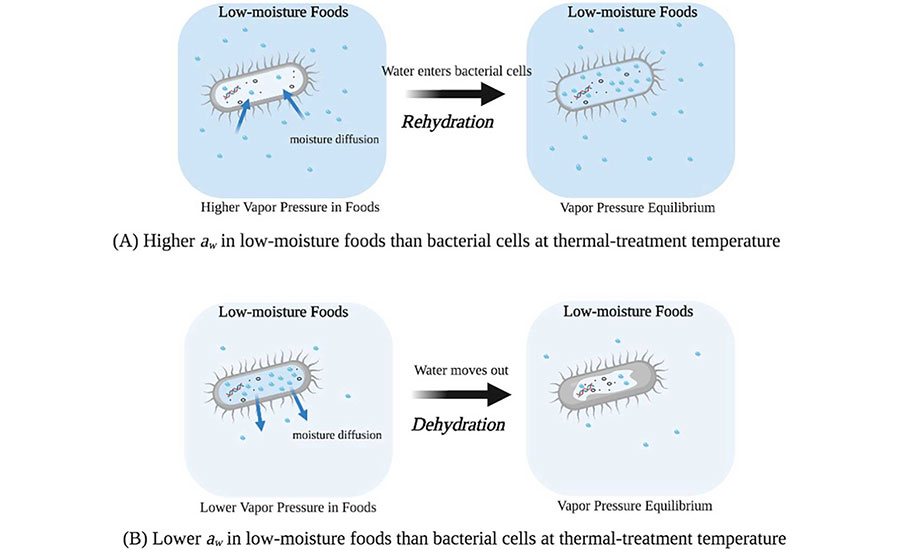
Figure 2 summarizes recently published data regarding changes in the water activity of different materials with temperature. The water activity of almond flour and wheat flour increases with temperature at different rates, while the water activity of peanut oil decreases with temperature. Figure 2 also includes the change in the water activity of the biomass of Salmonella cells. Salmonella demonstrates its own water activity trend that differs from the three food materials shown in the same figure. That is, bacterial cells gained moisture in wheat flour and lost moisture in almond flour and peanut oil when heated from room temperature to 80°C. As illustrated in Figure 3, the vapor pressure difference between bacterial cells and their surrounding environments will change the moisture content of the bacterial cells to reach an equilibrium with that of the environmental water activity. Such a process is estimated to take less than seconds.12 By measuring the high-temperature water activity of low-moisture foods and the D-value of Salmonella in a food matrix, it is possible to establish a quantitative relationship to predict the required pasteurization time at a specific temperature.10
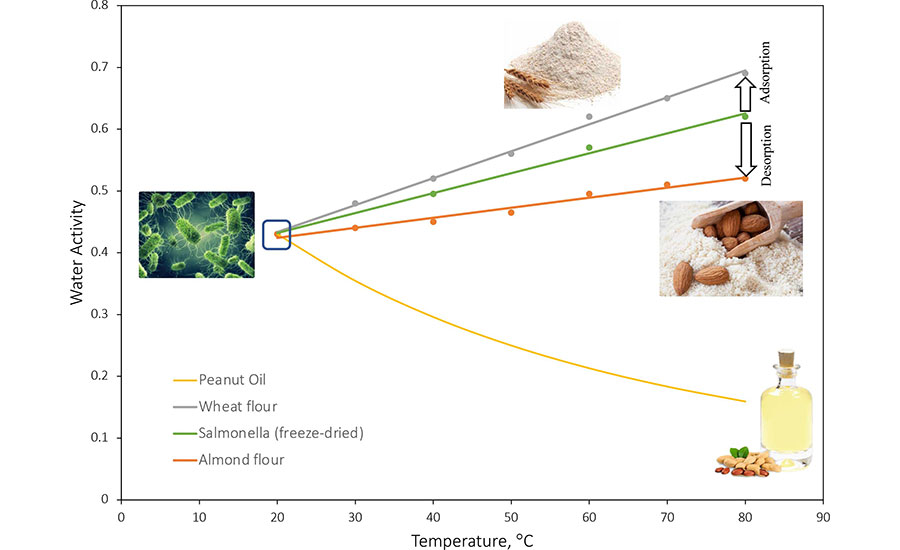
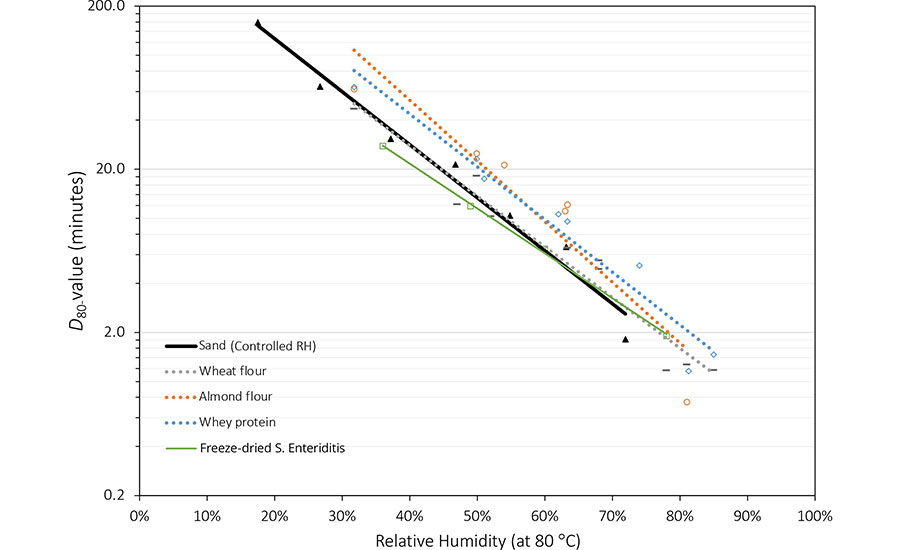
Several published studies reported quantitative relationships between D-values of Salmonella and high-temperature water activity of different low-moisture food matrices, including chocolate,14 peanut butter,15 dehydrated spices,16 egg powders,17 wheat flour, almond flour, and whey proteins.18 Figure 4 illustrates how the high-temperature water activity of the food matrix or the relative humidity during thermal treatment plays a significant role in determining the thermal resistance of Salmonella in low-moisture foods. These data can be utilized to predict the D-value of Salmonella in different low foods. For example, in the cases presented in Figure 2, when wheat flour with water activity of 0.43 is heated in sealed containers from 20 °C to 80 °C, the water activity of the material will change to 0.69. The D-value of Salmonella in the wheat flour at 80 °C will be 4.9 minutes. For almond flour, however, the water activity will change from 0.43 at 20 °C to 0.52 at 80 °C, and the corresponding D-value of Salmonella at 80 °C will be 21.2 minutes. For peanut oil, the water activity will change from 0.43 at 20 °C to 0.12 at 80 °C, and the D-value of Salmonella at 80 °C will be above 160 minutes.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
A mathematical equation describing the thermal resistance of a specific pathogen in low-moisture foods (adapted from Gaillard et al.20) can be used to help the design of effective thermal processing operations (Equation 1):
where Dref is the D value at the reference temperature, Tref, and the reference humidity, RHref; ZT and ZRH indicate the temperature and RH increase that leads to a tenfold reduction in D-value, respectively; MF is the matrix factor showing the effect of food composition.
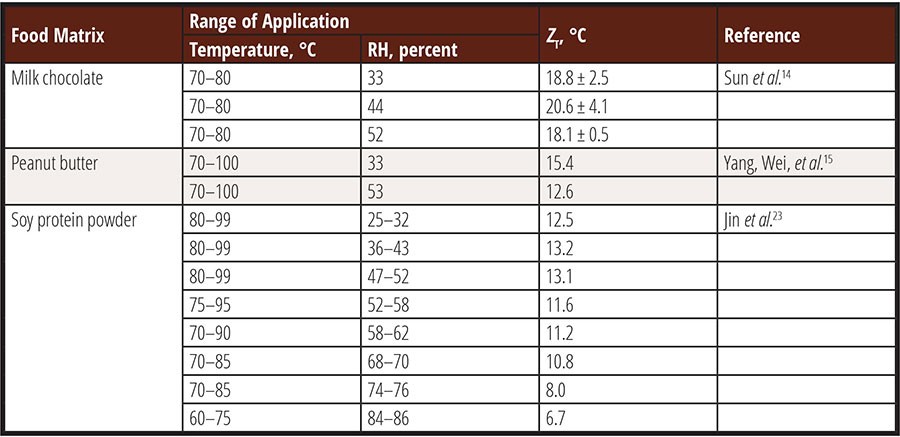
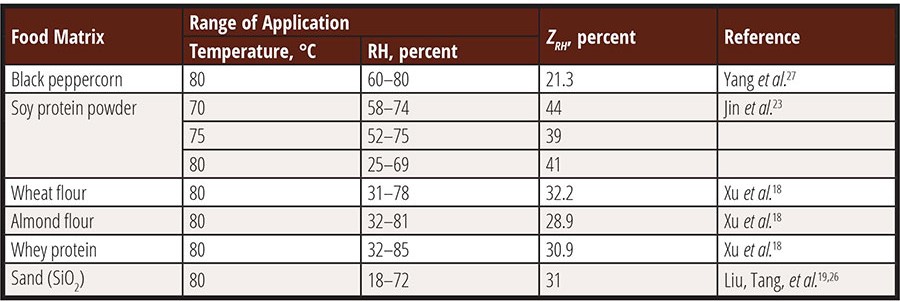
The reported ZT and ZRH values of Salmonella spp. are summarized in Table 1 and Table 2. The data reveals that ZT ranged between 6.7 °C and 20.6 °C, indicating a clear dependence on relative humidity where higher RH values correspond to lower ZT values. The lowest ZT value was observed in whey protein at 84–86 percent RH and 60–75 °C, measuring only 6.7 °C, which is merely 1–2 °C higher than the value reported for Salmonella in high-moisture meat products.21 Generally, ZT values for Salmonella spp. in low-moisture foods exceed 10 °C. These values are comparable to the ZT range of thermophilic spores in high-moisture foods (7–16 °C).22 This suggests that the thermal resistance of vegetative bacterial cells in low-moisture foods exhibits a similar sensitivity to temperature changes as that of spores in high-moisture foods.
ZRH indicates the increase in relative humidity required to cause a tenfold reduction in the D-value of Salmonella. Currently reported ZRH for Salmonella in low-moisture foods ranges between 21 percent and 41 percent at 80 °C. These values are higher than the ZRH value of Bacillus spores at 85–105 °C and 80–100 percent RH, which is 16 percent. It has been reported that ZRH may decrease significantly as RH surpasses 70–80 percent;23 however, further investigation is needed to validate this observation and provide guidance regarding the thermal death of bacteria at higher humidity levels.
Case Studies: Applying Valid Thermal Processing for Low-Moisture Foods Pathogen Control
Thermal processing of low-moisture foods involves intricate heat and mass transfer interactions among the food matrix, heat-conducting medium, microorganisms, and the surrounding gaseous phase. The actual temperature and humidity conditions experienced by microorganisms during thermal processing can vary due to factors such as heating method, equipment design, material properties (e.g., moisture content), and operational practices. To illustrate the practical implementation of our developed method for predicting microbial thermal resistance in low-moisture foods and designing effective thermal treatments, two case studies are presented below.
RF Heating for In-Package Pasteurization of Wheat Flour
To pasteurize wheat flour (e.g., in 5-lb bags) with different moisture contents (e.g., 11.6 percent and 14.5 percent) using an RF heating process (volumetric heating), the holding time required at a specific target temperature can be predicted by measuring or calculating the high-temperature water activities of the flour samples, and then determining their corresponding D-values for Salmonella. In this case, calculations indicate that the water activities at room temperature for the two batches of flour are 0.43 and 0.64, which would increase to 0.69 and 0.82, respectively, at 80 °C.24,25 According to the equation shown in Figure 4, the D80 values of Salmonella at these water activity levels are 3.2 and 1.2 minutes, respectively. To achieve a 5-log reduction, the two flour batches must be held at 80 °C for 16 and 6 minutes, respectively.
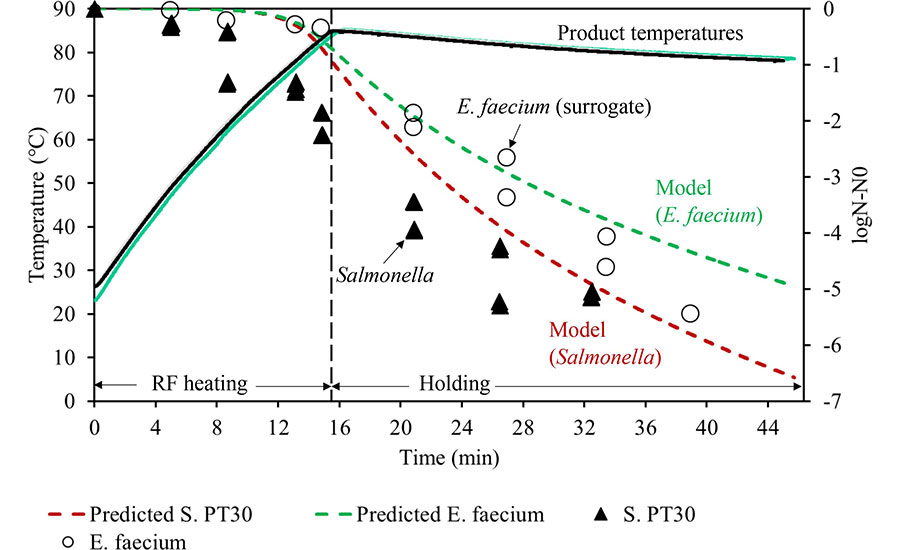
Alternatively, a higher temperature can be employed to reduce the holding time. For example, assuming a ZT value of 11 °C, the holding time can be reduced to 0.32 and 0.12 minutes if the sample is heated to 91 °C. Figure 5 illustrates an example of wheat flour pasteurization using RF heating and holding. The flour in the package (3 kg, aw = 0.45) was heated to 83 °C in 15 minutes and then held for a specific duration to achieve the desired level of lethality. As shown in Figure 5, the lethality contributed by the heating section can also be counted using a model to calculate the accumulated lethality (Equation 2).
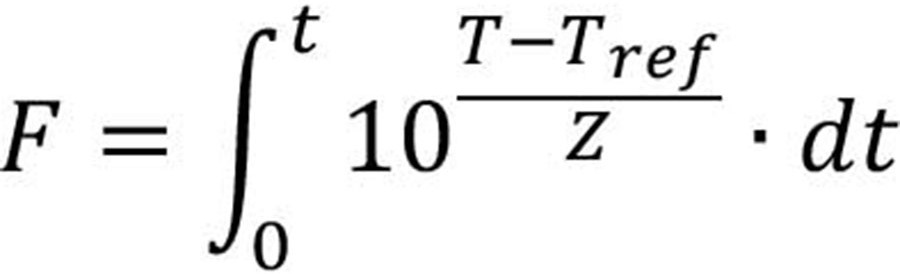
where F is the total equivalent heating time at the reference temperature; T is the real-time temperature at time t, and Dref is the D-value at the reference temperature, Tref. The log reduction in a process can be calculated as shown in Equation 3:
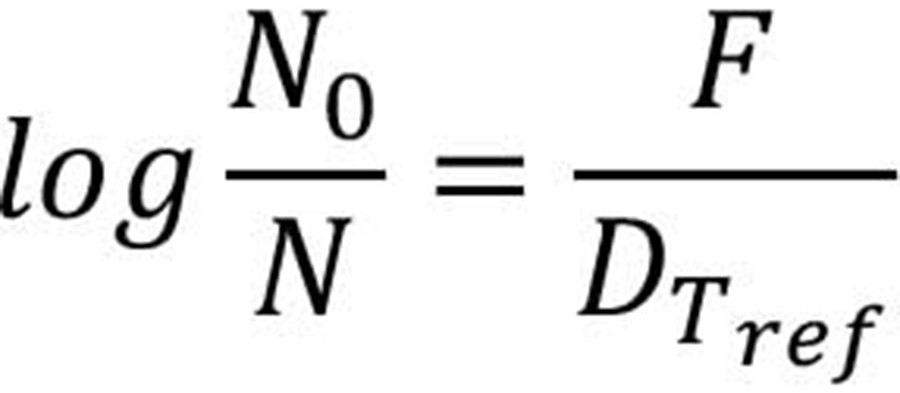
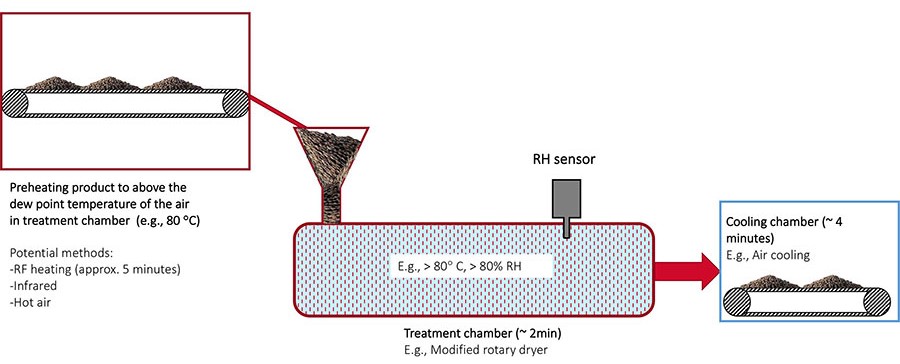
Controlled Humidity Thermal Pasteurization of Dehydrated Food Commodities
Our recent research has demonstrated the effectiveness of a highly efficient pasteurization process for low-moisture foods utilizing controlled humidity.27 In this process, low-moisture food commodities were preheated using conventional heating methods and subsequently transferred to a vessel with controlled temperature and humidity for a designated holding time (Figure 6). The purpose of preheating is to raise the product temperature above the dew point of the air in the treatment chamber, preventing condensation. After achieving the target level of lethality in the treatment chamber, the product was cooled using ambient air.
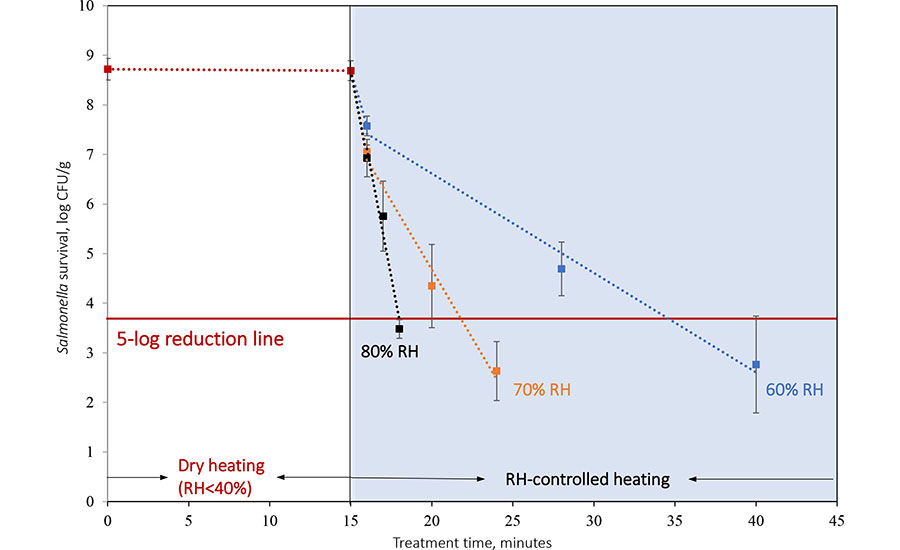
The authors' lab-scale unit has exhibited significant potential for pasteurizing various low-moisture food commodities (Figure 7). A 15-minute preheating (RH < 30 percent) resulted in negligible reduction of Salmonella, whereas the introduction of humid air (80 °C, RH between 60 percent and 80 percent) led to rapid bacterial reduction (a 6-log reduction in just 3 minutes at 80 percent RH). The reduction follows first-order kinetics, depending on temperature and humidity levels. This study provides a method for treating different low-moisture food commodities without the need to consider their initial moisture conditions. As discussed previously, humidity control plays a critical role in regulating the moisture content of bacteria on food particle surfaces. Preheating ensures that no condensation occurs when introducing humid hot air, potentially eliminating the need for post-treatment drying. By adjusting the combination of temperature and humidity, the treatment time can be tailored to preserve the quality of sensitive commodities.
Conclusion
Enhancing thermal processing methods for pathogen control in low-moisture foods is crucial for ensuring public health and the safety of food products. The thermal resistance of bacterial pathogens, particularly Salmonella, is influenced by various factors, including temperature, water activity, and relative humidity. Understanding these factors and their quantitative relationships enables the development of effective thermal treatments that can mitigate the risk of pathogen contamination in low-moisture food systems. The case studies in this article highlight the importance of considering high-temperature water activity and humidity control in designing valid thermal processes for low-moisture food pathogen control. By employing these scientific findings, the food industry can implement robust strategies to minimize the risk of outbreaks and recalls associated with low-moisture food commodities.
References
- Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO). Ranking of low moisture foods in support of microbiological risk management: Meeting report and systematic review. Microbiological Risk Assessment Series No. 26. Rome, 2022. https://doi.org/10.4060/cc0763en.
- Xu, J., D. H. Shah, J. Song, and J. Tang. "Changes in cellular structure of heat‐treated Salmonella in low‐moisture environments." Journal of Applied Microbiology 129, no. 2 (2020): 434–442. https://doi.org/10.1111/jam.14614.
- Li, M., G. Zhang, P. Gerner-Smidt, V. Mantripragada, I. Ezeoke, and M. P. Doyle. "Thermal Inactivation of Salmonella in Peanut Butter." Journal of Food Protection 72, no. 8 (2009): 1596–1601. https://doi.org/10.4315/0362-028X-72.8.1596.
- Potts, M. "Desiccation tolerance of prokaryotes." Microbiological Reviews 58 (1994): 755–805.
- Ball, P. "Water as an Active Constituent in Cell Biology." Chemical Reviews 108 (2008): 74–108. https://doi.org/10.1021/cr068037a.
- Davey, K. R. "Linear-Arrhenius models for bacterial growth and death and vitamin denaturations." Journal of Industrial Microbiology 12 (1993): 172–179.
- Arrhenius, S. "Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren." Zeitschrift für physikalische Chemie 4, no. 1 (1889): 226–248.
- Bigelow, W. "The logarithmic nature of thermal death time curves." The Journal of Infectious Diseases (1921): 528–536.
- Podolak, R., L. Lucore, and L. J. Harris. Heat Resistance of Salmonella and Other Bacterial Pathogens in Low Moisture Foods. Wiley, 2017. https://doi.org/10.1002/9781119071051.ch6.
- Syamaladevi, R. M., J. Tang, R. Villa-Rojas, S. Sablani, B. Carter, and G. Campbell. "Influence of Water Activity on Thermal Resistance of Microorganisms in Low-Moisture Foods: A Review." Comprehensive Reviews in Food Science and Food Safety 15, no. 2 (2016): 353–370. https://doi.org/10.1111/1541-4337.12190.
- Garces-Vega, F. J., E. T. Ryser, and B. P. Marks. "Relationships of Water Activity and Moisture Content to the Thermal Inactivation Kinetics of Salmonella in Low-Moisture Foods." Journal of Food Protection, 82, no. 6 (2019): 963–970. https://doi.org/10.4315/0362-028x.jfp-18-549.
- Syamaladevi, R. M., J. Tang, and Q. Zhong. "Water Diffusion from a Bacterial Cell in Low-Moisture Foods." Journal of Food Science 81, no. 9 (2016): R2129–R2134. https://doi.org/10.1111/1750-3841.13412.
- Xie, Y., J. Xu, R. Yang, J. Alshammari, M. J. Zhu, S. Sablani, and J. Tang. "Moisture content of bacterial cells determines thermal resistance of Salmonella enterica serotype Enteritidis PT 30." Applied and Environmental Microbiology 87, no. 3 (2021): e02194-20. https://doi.org/10.1128/AEM.02194-20.
- Sun, S., Y. Xie, R. Yang, M.-J. Zhu, S. Sablani, and J. Tang. "The influence of temperature and water activity on thermal resistance of Salmonella in milk chocolate." Food Control 143 (2023): 109292. https://doi.org/10.1016/j.foodcont.2022.109292.
- Yang, R., L. Wei, J. Dai, and J. Tang. "Thermal death kinetics of Salmonella Enteritidis PT30 in peanut butter as influenced by water activity." Food Research International 157 (2022): 111288. https://doi.org/10.1016/j.foodres.2022.111288.
- Xie, Y., S. Zhang, S. Sun, M.-J. Zhu, S. Sablani, and J. Tang. "Survivability of Salmonella and Enterococcus faecium in chili, cinnamon and black pepper powders during storage and isothermal treatments." Food Control 137 (2022): 108935. https://doi.org/10.1016/j.foodcont.2022.108935.
- Pérez-Reyes, M. E., X. Jie, M.-J. Zhu, J. Tang, and G. V. Barbosa-Cánovas. "Influence of low water activity on the thermal resistance of Salmonella Enteritidis PT30 and Enterococcus faecium as its surrogate in egg powders." Food Science and Technology International 27, no. 2 (2021): 184–193.
- Xu, J., J. Tang, Y. Jin, J. Song, R. Yang, S. S. Sablani, and M.-J. Zhu. "High temperature water activity as a key factor influencing survival of Salmonella Enteritidis PT30 in thermal processing." Food Control 98 (2019): 520–528. https://doi.org/10.1016/j.foodcont.2018.11.054.
- Liu, S., J. Tang, R. Tadapaneni, R. Yang, and M.-J. Zhu. "Exponentially Increased Thermal Resistance of Salmonella spp. and Enterococcus faecium at Reduced Water Activity." Applied and Environmental Microbiology 84, no. 8 (2018): e02742-17. https://doi.org/10.1128/aem.02742-17.
- Gaillard, S., I. Leguérinel, and P. Mafart. "Model for combined effects of temperature, pH and water activity on thermal inactivation of Bacillus cereus spores." Journal of Food Science 63, no. 5 (1998): 887–889.
- McCormick, K., I. Y. Han, J. C. Acton, B. W. Sheldon, and P. L. Dawson. "D and z-values for Listeria monocytogenes and Salmonella Typhimurium in packaged low-fat ready-to-eat turkey bologna subjected to a surface pasteurization treatment." Poultry Science 82, no. 8 (2003): 1337–1342. https://doi.org/10.1093/ps/82.8.1337.
- Da Silva, N., M. H. Taniwaki, V. C. Junqueira, N. Silveira, M. M. Okazaki, and R. A. R. Gomes. Microbiological Examination Methods of Food and Water: A Laboratory Manual. 2nd Ed. CRC Press, 2018.
- Jin, Y., J. Tang, and M.-J. Zhu. "Water Activity Influence on the Thermal Resistance of Salmonella in Soy Protein Powder at Elevated Temperatures." Food Control 113 (2020): 107160. https://doi.org/10.1016/j.foodcont.2020.107160.
- Tadapaneni, R. K., R. Yang, B. Carter, and J. Tang. "A new method to determine the water activity and the net isosteric heats of sorption for low moisture foods at elevated temperatures." Food Research International 102 (2017): 203–212. https://doi.org/10.1016/j.foodres.2017.09.070.
- Tadapaneni, R., R. M. Syamaladevi, R. Villa-Rojas, and J. Tang. "Design of a novel test cell to study the influence of water activity on the thermal resistance of Salmonella in low-moisture foods." Journal of Food Engineering 208 (2017): 48–56. https://doi.org/10.1016/j.jfoodeng.2017.03.025
- Liu, S., S. Ozturk, J. Xu, F. Kong, P. Gray, M.-J. Zhu, S. S. Sablani, and J. Tang. "Microbial validation of radio frequency pasteurization of wheat flour by inoculated pack studies." Journal of Food Engineering 217 (2018): 68–74. https://doi.org/10.1016/j.jfoodeng.2017.08.013.
- Yang, R., S. P. Lombardo, W. F. Conway, and J. Tang. "Inactivation of Salmonella Enteritidis PT30 on black peppercorns in thermal treatments with controlled relative humidities." Food Research International 162 (2022): 112101. https://doi.org/10.1016/j.foodres.2022.112101.
Ren Yang, Ph.D., is a Postdoctoral Research Associate at the Department of Biological Systems Engineering, Washington State University in Pullman, Washington. His research interests include sustainable, systems-based solutions for ensuring low-moisture food safety; the use of RF preheating of cold, high-moisture particulate biomaterials to reduce drying time; the accuracy and durability of existing humidity sensors at conditions of high temperature and high humidity; and design of a controllable pasteurization process that can be easily validated for multiple low-moisture foods. He holds a Ph.D. in Food Engineering from Washington State University, a M.S. degree in Food Safety and Technology from the Illinois Institute of Technology, and a B.Eng. degree in Food Science and Engineering from Northwest University.
Juming Tang, Ph.D., is Regents Professor and Distinguished Chair of Food Engineering in the Department of Biological Systems Engineering at Washington State University in Pullman, Washington. He holds several patents and numerous awards and honors for his research in microwave pasteurization, microwave sterilization of food, and low-moisture food safety. He is currently the principal investigator of a multi-institutional team represented by members of industry, academia, and government investigating engineering solutions to ensure microbial safety of frozen and refrigerated meals in retail markets. He is also the Director of the Microwave Sterilization Consortium, again represented by members of industry, academia, and government, that was awarded the first-ever industrial microwave sterilization process approval from the U.S. Food and Drug Administration (FDA). He holds a Ph.D. in Agricultural/Food Engineering from the University of Saskatchewan in Canada, an M.S. degree in Agricultural/Food Engineering from the University of Guelph, and a B.S. degree in Mechanical Engineering from South Central China University.
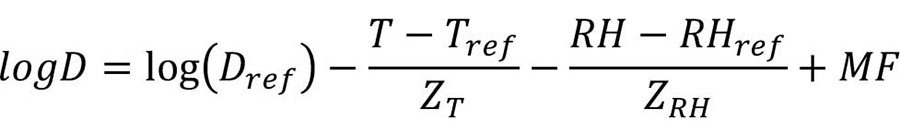







.webp?t=1721343192)
