Codex Principles of Food Hygiene 3.0
A third revision to Codex’s General Principles of Food Hygiene is in progress.

The Codex Committee on Hygiene is in the process of revising the General Principles of Food Hygiene (Codex, 2020). This document was first published in 1969; so far, six major changes have been made to the document including revisions and amendments.1,2,3 As of the writing of this article, another revision is currently in progress.3 Codex has published the body of the text and most of the diagrams. However, the decision tree is still under review by the Codex Committee.
The revision can be described in the following way:
- A new look
- A reformatting of the clauses within the text
- Expansion of some existing guidance
- Several new clauses.
The document addresses the Good Hygiene Practices (GHPs) and Hazard Analysis and Critical Control Points (HACCP) requirements for food businesses. Codex states the following objectives for the GHP and HACCP system:
- “Provide principles and guidance on the application of GHPs applicable throughout the food chain to provide food that is safe and suitable for consumption”
- “Provide guidance on the application of HACCP principles”
- “Clarify the relationship between GHPs and HACCPa”
- “Provide the basis on which sector and product-specific codes of practice can be established” (Codex, 2020).
The document is written from a perspective that assumes a food business organization is implementing GHPs and HACCP. In addition, GHP and HACCP requirements are written with sufficient flexibility so that they can be applied to organizations throughout the food chain, including small businesses. Guidance is provided to aid in the development of a food safety system for small or less developed businesses. Flexibility is achieved by taking into account the nature of the business, financial resources, infrastructure, processes, knowledge, and competencies of employees, and food safety risk associated with the product. The goal is the development of a food safety system that assures the production of safe food. The first step in developing a food safety system is the implementation of prerequisite programs, including GHPs. The prerequisite programs must be well-established, operational, and verified.
The document is also intended to be used by regulatory authorities in developing and implementing food safety regulations and guidance. HACCP was formally incorporated into the document rather than being present in an annex.
Small but Important Revisions
First, all of the requirements in the 2003 revision have been included in the 2020 edition. One change is that Codex has reorganized the clauses. Some individuals may say that this is just “rearranging the chairs”; however, the new format assembles the clauses in a more logical format, which should make it easier for users to understand and apply the concepts presented within the document. The GHP chapter of the document retains the traditional format. Each major clause starts with a text box that describes both the objective and the rationale for the clause.
The General Principles of Food Hygiene is being published in English, French, Spanish, Arabic, Chinese, and Russian. The 2020 version offers more in-depth guidance to provide the food safety professional with a better understanding of the principles of food hygiene. The side bar presents a comparison of the 2003 version to the 2020 version of the document.
Major Revisions in HACCP
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
The document addresses food safety culture. The focus is on management’s responsibilities for creating a positive food safety culture for all employees in the organization. The standard addresses the following issues in developing and ensuring a food safety culture:
- “Commitment of the management and all personnel to the production and handling of safe food”
- “Leadership to set the right direction and to engage all personnel in food safety practices”
- “Awareness of the importance of food hygiene by all personnel in the food business”
- “Open and clear communication among all personnel in the food business, including communication of deviations and expectations”
- “The availability of sufficient resources to ensure the effective functioning of the food hygiene system” (Codex, 2020).
In 2008, Codex published Guidelines for the Validation of Food Safety Control Measures. In this guideline, Codex describes validation, monitoring, and verification as separate activities. Validation determines whether a control measure is capable of achieving its purpose of controlling a hazard(s). Validation should be done prior to full implementation of a process. Monitoring and verification take place after a process has been implemented. Monitoring is designed to provide real-time information to determine if a process is operating within specified limits and to provide information on process performance. Verification can occur during or after the operation of a control measure. It is designed to determine if the control measure is operating according to its design.
The U.S. Department of Agriculture’s Food Safety and Inspection Service (USDA-FSIS) has published an excellent guideline on the topic of validation (FSIS, 2015).4 Although the guideline is primarily for smaller meat and poultry establishments, the principles can be applied throughout the entire food chain. FSIS’s approach is to divide the design of the HACCP system into two parts:
- The design phrase, where the principles of food safety are designed into the process. Ideally, this phase should be conducted prior to manufacturing the food.
- The execution phase, where the manufacturing process is validated to ensure that the manufacturing process can achieve the objective of the HACCP or food safety plan.
Additional information on the validation of HACCP and food safety systems can be found in the archives of Food Safety Magazine (e.g., Surak and Steir, 20095; Surak, 20156; and Surak and Steir, 20177).
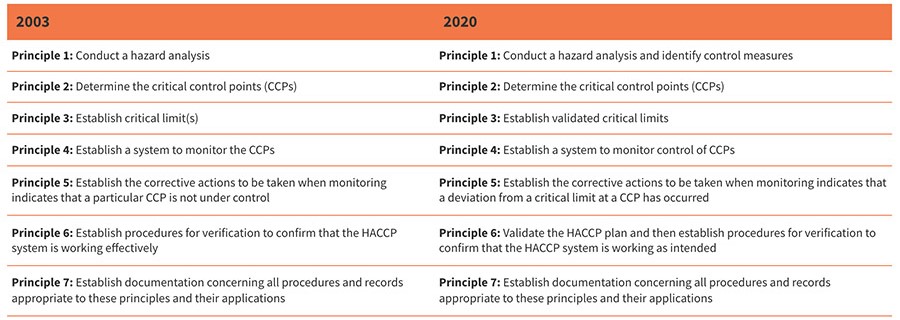
The 2020 version of the General Principles of Food Hygiene has incorporated the concepts of validation, monitoring, and verification. This has been accomplished both by renaming the seven principles of HACCP (Table 1) and by providing increased guidance in the following clauses:
- 3.8 Establish validated critical limits for each CCP (Step 8/Principle 3)
- 3.11 Validation of the HACCP plan and verification procedures (Step 11/Principle 6).
Guidance on Step 6/Principle 1 has been expanded. This step will address the following activities:
- List all potential hazards that are likely to occur and associated with each step in the process
- Conduct a hazard analysis to identify significant food safety hazards
- Consider any control measures to control identified hazard(s).
TABLE 1. Comparison of 2003 and 2020 Versions of HACCP from the Principles of HACCP. Sources: Codex, 2003 and 2022
Annex and Diagrams
At the writing of this article, the annex contains the following sections:
- A table that compares control measures applied at a GHP with a control measure applied at a CCP
- A diagram that shows the 12 steps of Codex HACCP
- An example of a hazard analysis worksheet, similar to the Form 2-B: FSPCA Form for Hazard Analysis (FDA, 2018)8
- An example of a hazard analysis worksheet, equivalent to Form 2-C: FSPCA Form for Process Controls (FDA, 2018); some individuals will title this worksheet “HACCP Summary Form.”8
A decision tree is expected to be added to the final edition of the document.
Table of Contents
At present, the document does not contain a table of contents. Table 2 shows an outline of the clauses in the 2020 revision.b
TABLE 2. Current Structure of the General Principles of Food Hygiene
References
- Codex. 2003. Recommended International Code of Practice. General Principles of Food Hygiene. CAC/RCPP 1-1969, Rev. 4-2003. Codex Alimentarius. FAO, Rome, Italy. https://www.mhlw.go.jp/english/topics/importedfoods/guideline/dl/04.pdf and https://www.mhlw.go.jp/english/topics/importedfoods/guideline/dl/05.pdf.
- Codex. 2008. Guidelines for the Validation of Food Safety Control Measures. CAC/GL69-2008. Codex Alimentarius. FAO, Rome, Italy. http://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/tr/.
- Codex. 2020. General Principles of Food Hygiene. CXC-1-1969. Codex Alimentarius. FAO, Rome, Italy. http://www.fao.org/fao-who-codexalimentarius/codex-texts/codes-of-practice/en/.
- USDA-FSIS. 2015. FSIS Compliance Guideline: HACCP System Validation. https://www.fsis.usda.gov/guidelines/2015-0011.
- Surak, J.G. and R.F. Steir. 2009. “Validating Food Safety Controls.” Food Safety Magazine. https://www.food-safety.com/articles/3907-validating-food-safety-controls.
- Surak, J.G. 2015. “A New Paradigm for Validation, Verification and Monitoring.” Food Safety Magazine. https://www.food-safety.com/articles/3660-a-new-paradigm-for-validation-verification-and-monitoring.
- Surak, J.G. and R.F. Steir. 2017. “Validation 2.0.” Food Safety Magazine. https://www.food-safety.com/articles/5320-validation-20.
- FDA. 2018. Draft Guidance for Industry: Hazard Analysis and Risk-Based Preventive Controls for Human Food: Appendix 2. Washington, D.C. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-hazard-analysis-and-risk-based-preventive-controls-human-food.
Notes
a. One editorial way this has been done is that HACCP is formally incorporated into the document rather than being present as an annex. Thus, chapter 1 now contains the GHPs, and chapter 2 details HACCP.
b. The 2020 version of the standard does not include a table of contents.
John G. Surak, Ph.D., is the Principal of John Surak and Associates. He provides consulting on food safety and quality management systems, auditing management systems, validating manufacturing processes, designing and implementing process control systems, and implementing Six Sigma and business analytics. Dr. Surak is a member of the Editorial Advisory Board of Food Safety Magazine. His website is www.stratecon-intl.com/jsurak.html, and he can be reached at jgsurak@yahoo.com.









