FDA’s Office of Food Additive Safety
The U.S. Food and Drug Administration (FDA) has regulatory oversight for substances added to food, including monitoring their safe use. Under the Federal Food, Drug, and Cosmetic Act (FDCA), FDA must review the safety of food and color additives before manufacturers and distributors can market them. To initiate this review, sponsors are required to submit a petition or notification that includes appropriate test data to demonstrate the safety of the intended use of the substance. The agency also has a notification program for substances that are "Generally Recognized As Safe" (GRAS). Finally, developers of foods derived from bioengineered plants consult with FDA to ensure that all safety and regulatory questions are resolved prior to marketing.
The newly formed Office of Food Additive Safety (OFAS) within FDA's Center for Food Safety and Applied Nutrition (CFSAN) is FDA's one-stop shop for questions about the safety of food ingredients, food packaging and food processing equipment, including sources of radiation used to treat or inspect food, and foods derived from bioengineered plants. OFAS is the lead for FDA's food and color additive petition processes, the consideration of independent determinations of GRAS status, and review of notifications for food contact substances.
The new structure of OFAS reflects the tremendous changes that the food ingredient review program has undergone in the past decade. These changes include the proposal in 1997 to replace the GRAS affirmation petition process with a GRAS notice procedure, and the statutory provisions of the Food and Drug Administration Modernization Act of 1997 (FDAMA) that created the food contact substance notification process.
OFAS, the former Office of Premarket Approval (OPA), is structured based on its review responsibilities. The new structure consists of four divisions, three of which are focused on particular types of reviews—the Division of Petition Review, the Division of Food Contact Substance Notification Review, and the Division of Biotechnology and GRAS Notice Review. The fourth, the Division of Chemistry Research and Environmental Review, includes the OFAS's laboratory groups and its environmental review group. There also are two distinct organizational units immediately under the OFAS Director: the Office Operations Staff and the Staff of the Associate Director for Science and Policy. The Office Operations Staff oversees most of the administrative matters within the office, including correspondence and Freedom of Information (FOI) requests. The Associate Director for Science and Policy supervises a staff of the senior office personnel in the areas of regulatory science and policy.
OFAS's new structure is partially a result of a total of $11.4 million of additional funding that was provided starting in Fiscal Year (FY) 2000 to improve the food additive safety review program by increasing the efficiency of the submission and review process. Importantly, the new funding has made it possible to hire review and regulatory experts in the various review areas. The new structure of OFAS has a number of advantages, among which are clearer delineation of responsibility among the review divisions based on the regulatory status of a food ingredient or submission type. In addition, each of the three divisions with specific review responsibilities contains the majority of the resources necessary to complete its assigned reviews. Thus, accountability and oversight for the various review processes is increased. One result of the increase in review resources is the greater ability for each review division to actively work with industry prior to providing their data submissions to the agency and to provide improved guidance to potential petitioners through a variety of means, including the website.
Over the past few years OFAS has made most of its guidance available via the Internet. Efforts are underway to create a "paperless" office by establishing an electronic submission capability. Currently, food and color additive petitions may be submitted in this manner. The Food Additive Regulatory Management (FARM) system, which makes documents under review in the office available to all employees electronically, also provides a modern tracking system for the reviews.
The Division of Petition Review
The Division of Petition Review (DPR) oversees the heart and soul of FDA's premarket review program for food ingredients, the safety review of the food and color additive petition. This division is charged with the premarket review of direct food and color additive petitions, such as new sweeteners and antimicrobials for meats, fruits and vegetables. In addition, the color additive petition review program in DPR performs the safety review for color additives used in all FDA-regulated applications, including foods, cosmetics, drugs and medical devices. DPR has benefited from the restructuring to bring those individuals most experienced in the review of food and color additive petitions under one managerial umbrella.
The creation of DPR is the latest step in an ongoing process of streamlining the food and color additive petition review process. This effort began in earnest in mid-1995 when FDA committed to dramatically reduce the inventory of food and color additive petitions and to meet realistic and predictable time frames for reaching decisions on newly submitted petitions. Since 1995, the office has reduced its inventory of food and color additive petitions under review from more than 200 to fewer than 25. In addition to the initial inventory in 1995, FDA also received about 300 additional petitions during the above period, so the true number of petitions processed since mid-1995 is approximately 500. Many efforts have contributed to this outcome, including the statutory and regulatory changes listed above, the creation of specialized review groups, the codification of a threshold of regulation policy, and the use of contract reviews to augment agency resources.
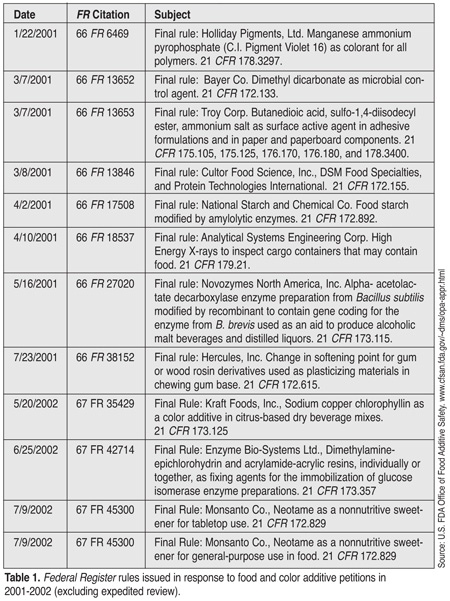
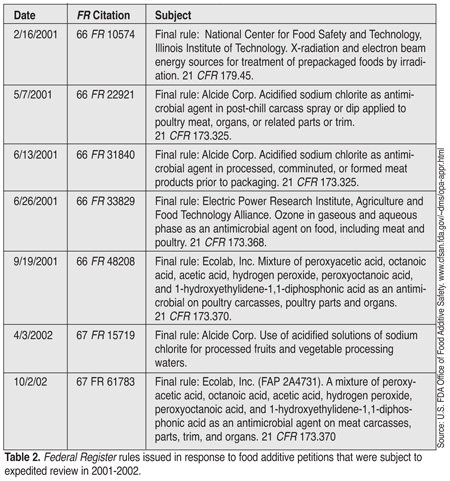
Among the specialized responsibilities of DPR is oversight of FDA's expedited review process for food additives that are expected to have a significant impact on food safety. For example, a number of antimicrobials used in processing of meat and poultry have been evaluated under this program initiated in 1998 in response to growing concerns regarding the microbial contamination of food (Tables 1 and 2). To date, the program has shepherded the review of 23 food additive petitions with an average response rate on the order of 180 days. Since its inception, the performance of this program has been a high priority for CFSAN.
The Division of Food Contact Substance Notification Review
The creation of the food contact notification (FCN) process by FDAMA and the resources made available to FDA to implement that program drove the creation of a new division to implement it. The committee that developed the regulations and guidance for the FCN program also recommended the creation of a separate review division for food contact notifications.
Prior to the creation of the FCN program, all of the food contact substances, which were informally called "indirect" food additives, had to undergo the same premarket approval process as those compounds added directly to food. Thus, components of food packaging that become components of food at only very low levels were authorized in the same way as artificial sweeteners, antioxidants and color additives. Since the passage of the food additives amendment to the FDCA in 1958, FDA and its regulated industry have both felt that there might be a more efficient way to regulate food contact substances. Over the years, FDA made several improvements in the review program for food contact substances that were possible within the confines of the existing statute. These included a "threshold of regulation policy" which was based on a de minimis legal theory, on an evaluation of available toxicity data of representative chemical compounds, and with a "special project team" (SPT) that expedited the review of the most routine indirect food additive petitions. The work of the SPT and threshold of regulation teams laid the groundwork for the successful implementation of the FCN program that now inventories more than 235 effective notifications. An FCN becomes effective 120 days from receipt of a complete notification for the substance unless the agency objects.
The Division of Biotechnology and GRAS Notice Review
This division conducts consultations with industry in regard to the safety and regulatory status of bioengineered foods. It evaluates notices that are submitted in support of a sponsor's conclusion that a substance is not subject to premarket review and approval because it is GRAS.
In the Federal Register of May 29, 1992 (57 FR 22984), FDA published its "Statement of Policy: Foods Derived from New Plant Varieties." The 1992 policy clarified the agency's interpretation of the application of the FDCA with respect to human foods and animal feeds derived from new plant varieties and provided guidance to industry on scientific and regulatory issues related to these foods. In the 1992 policy, FDA recommended that developers consult with FDA about bioengineered foods under development; since then, developers have routinely done so.
The process for such consultations often begins relatively early in the development of the bioengineered plant products and consists of an iterative exchange of information. OFAS guides the sponsor through the development and evaluation of appropriate data to ensure that all food safety questions have been addressed. To date, FDA has completed consultations with industry on more than 50 bioengineered plant products. A list of the completed consultations can be found on the OFAS web site. In January 2001, FDA issued a proposal to establish a mandatory premarket notification program for bioengineered foods from new plant varieties. FDA received numerous comments on the proposed notification process; these are receiving careful consideration by the agency.
A substance that will be added to food is subject to premarket approval by FDA unless its use is prior sanctioned or determined GRAS by qualified experts. On April 17, 1997, FDA issued a proposed rule (62 FR 18938) that would establish a notification procedure whereby any person may notify FDA of a determination by that person that a particular use of a substance is GRAS. As described in the GRAS proposal, the agency evaluates whether each submitted notice provides a sufficient basis for a GRAS determination and if information in the notice or otherwise available to FDA raises issues that lead the agency to question whether use of the substance is GRAS.
Following evaluation, FDA responds to the notifier by letter, generally in one of three ways: (1) the agency does not question the basis for the notifier's GRAS determination; (2) the agency concludes that the notice does not provide a sufficient basis for a GRAS determination (e.g., because the notice does not include appropriate data, or because the available data raise questions about the safety of the notified substance); or (3) the agency has, at the notifier's request, ceased to evaluate the GRAS notice. Between February 1998 and September 2002, FDA received 115 GRAS notices and responded with an average response time of 160 days.
The Division of Chemistry Research and Environmental Review
This division within OFAS encompasses two groups of scientists--the chemistry laboratory groups and the environmental review group. The laboratory staff includes both a food contact substance and direct additive group that performs research and specialized reviews in support of OFAS's premarket review activities. These laboratory groups have conducted research on many food safety issues related to food packaging and direct additives such as bromates in bakery products, migration from food packaging and recycled materials, endocrine disruptors in food and food packaging, and irradiation of food packaging and foods.[1-5]The environmental staff has the responsibility for reviewing all submissions to CFSAN in accordance with the National Environmental Policy Act.
Other OFAS Activities
While the OFAS focus is assuring the safe use of ingredients used in traditional foods and of substances used in food packaging, OFAS scientists support many other CFSAN programs, including those responsible for the review of dietary supplements and infant formulas. Through work with the National Academy of Sciences, OFAS scientists oversee efforts to create standards for food ingredients in the U.S. and work to set international standards through Codex Alimentarius. In sum, the OFAS program in CFSAN is an integral part of the agency's mission to protect public health.
Mitchell A. Cheeseman, Ph.D., is the Director of the Division of Food Contact Substance Notification Review in OFAS. He has worked at FDA in the areas of food and color additives and GRAS substances since 1991.
James C. Wallwork, Ph.D., is involved in the review of food and color additive petitions as an acting supervisory consumer safety officer in the OFAS Division of Petition Review.
References
1. Diachenko, G. and C. Warner. Chapter 16, Potassium Bromate in Bakery Products: Food Technology, Toxicological Concerns, and Analytical Methodology. In Bioactive Compounds in Foods-Effects of Processing and Storage. Symposium Series 816. American Chemical Society. 2002.
2. Begley, T. Migration from food packaging: regulatory considerations for estimating exposure. In Plastic Packaging Materials for Food. John Wiley-VCH. 2000.
3. Begley, T.H., et al. Evaluating the potential for recycling all PET bottles into new food packaging. Food Addit. and Contam., (19):135-143. 2002.
4. McNeal, T.P., et al. Determination of suspected endocrine disruptors in food and food packaging. Series 747. American Chemical Society. 1999.
5. Buchalla, R., et al. Analysis of low-molecular weight radiolysis products in extracts of gamma-irradiated polymers by gas chromatography and high-performance liquid chromatography. Radiation Phys. and Chem. (63):837-840. 200
>
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →







