The Interagency Risk Assessment Consortium: Improving Coordination Among Federal Agencies
Risk assessment, when applied to food safety, is the scientific evaluation of potential adverse health effects that result from human exposure to hazards. It can play an important role in policy development, enabling government agencies and those in the food industry to further increase the safety of the food supply and improve public health.
Given the importance of risk assessment to public health decisions, it is only logical that federal food safety agencies have an established mechanism to engage stakeholders, academia, and each other on current risk assessment issues. This is accomplished through an interagency Risk Assessment Consortium (RAC). The RAC was established to improve food safety by improving coordination of activities among federal agencies. Additionally it offers interested parties outside the federal government an opportunity to participate in discussions on risk analysis issues with federal agencies through attending discussions and question-and-answer sessions with national and international risk assessment experts. This article describes how the RAC strives to improve coordination among member agencies to benefit not only the agencies but also stakeholders and the academic community across the food safety spectrum.
Building the RAC
The RAC, whose membership consists of federal agencies with food safety responsibilities or interest, was formed at the outset of the 1997 Food Safety Initiative. The Food Safety Initiative created a resurgence of emphasis on collaboration between regulators and stakeholders, between federal agencies and states, and between the very agencies charged with assessing the risks posed by contaminants in the food supply. Coordination of risk assessment activities among federal agencies is critical for improving food safety decision-making. The RAC is charged with working collaboratively among the member agencies to promote the conduct of scientific research that will facilitate risk assessments; identify, catalog and provide broad access to risk assessment information; and serve as a resource for all its member agencies.[1]
Since its inception, the RAC has steadily built recognition as an essential element of effective interagency coordination and has participated in many of the advances in the field of microbiological risk assessment. In 1997, it was questionable as to whether meaningful microbial risk assessments could be conducted. There are many factors that potentially play a role in foodborne illness, and at the time there was uncertainty about whether a risk assessment could properly identify the conditions under which food contaminated with microbial pathogens likely would result in human illness. The type of pathogen in question, the type of food, the initial microbial load, the multiplication rate of the pathogen, the type of processing and packaging the food underwent, the storage conditions (time and temperature), cooking methods, and susceptibility of different at-risk populations are all variables that are considered in a risk assessment.
In an effort to address the many challenges faced by microbiological risk assessors, the RAC sponsored or co-sponsored numerous meetings and workshops at which food safety scientists could network and share information. At these meetings research and information needs were discussed, techniques for using data were examined, means for predicting human dose-response relationships from multiple biological models were addressed, and more effective information sharing and communication was explored. These efforts helped enhance the predictive capabilities of microbiological risk assessments that are now routinely used in decision-making in the U.S. and an increasing number of countries worldwide. Building on its successes with microbiological risk assessment issues, the RAC is increasingly involved in topical cross-cutting issues that confront the risk assessment community, including chemical and nutritional risk assessments. The RAC currently includes membership from 18 federal agencies or sub-agencies (Table 1)..jpg)
The RAC addresses the types of questions that confront federal agencies almost every day in making regulatory decisions: Are the data good enough? Should we use the data? Are we too uncertain to propose a solution? How should our work be reviewed? Where can we find more data and resources? The RAC facilitates a collaborative approach to address these questions, with a beneficial impact for all the agencies and the food safety risk analysis community.
It All Comes Down to Better Communication
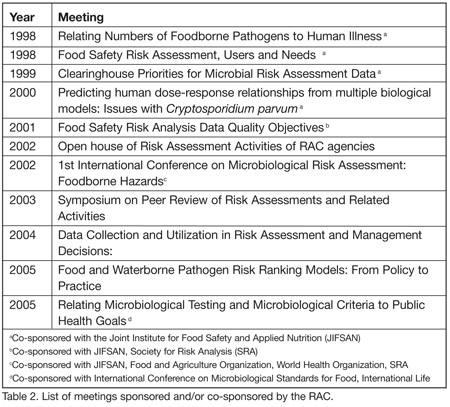
Better communication and coordination of risk assessment activities is essential for improved food safety decision-making. Networking and collective thinking through our workgroups has allowed the RAC to anticipate key issues and bring them to the national stage. Initially the RAC gathers information, then solicits input from stakeholders in public fora, and finally prepares summaries to frame the issues. The public fora include workshops, symposia, and conferences (Table 2). The summaries are shared with decision makers and stakeholders through web publication, journal proceedings and presentations. Some of the more significant RAC activities are described as follows:
Accessing Data: The Food Safety Risk Analysis Clearing House. The limited availability of data is one of the common themes that concerns both risk assessors and decision makers. Deter-mining what data are available is an early step in any risk assessment. The Food Safety Risk Analysis Clearinghouse (foodrisk.org) was established with the primary purpose of being a repository for data from governments, industry, and academic researchers. Such data would then be available to everyone, in a transparent way, to assist them in their risk assessment and risk analysis efforts. The RAC has had a long, close relationship with the Joint Institute for Food Safety and Applied Nutrition (JIFSAN), which in collaboration with the RAC Clearinghouse workgroup, launched the Clearinghouse effort in 1998. JIFSAN, a collaboration between the University of Maryland and the U.S. Food and Drug Administration (FDA), still manages the Clearinghouse today. The Clearinghouse has grown and evolved into a widely used portal for food safety professionals looking for data, information, completed risk assessments, research needs, tutorials, and current events. The RAC uses the Clearinghouse to widely disseminate quarterly meeting minutes, our annual plan, upcoming RAC-sponsored workshops and symposia, and materials and presentations from these activities.
Using Data: Data Quality and Data Utility. Risk assessors must typically search for any available data and information to address the specific questions posed by risk managers. The old adage “garbage in, garbage out” also applies to risk assessments. In addition to the challenging task of identifying data sources, risk analysts must also be concerned about the quality of the data and the utility of the data. Data quality considerations include objectivity, utility, and integrity of information. Data utility refers to factors like the age of the data (i.e., are 10-year-old data still representative of today’s situation?); the country or region of collection (i.e., are conditions similar enough between the Pacific Northwest and the Gulf of Mexico?); and transferences that must be made (i.e., would data on oranges be appropriately used for tangerines?).
To help answer such questions, the RAC sponsored a public meeting in 2001 on data quality objectives, establishing guidelines for the Clearinghouse data users. A second workshop in 2004 focused on data collection and utilization where some of the characteristics of good data and the challenges risk assessors and data generators face to provide useful input to risk assessments were identified. The RAC continues to collect information on data utility approaches from the broader risk analysis community to provide guidance to member agencies that must concern themselves with these issues. It also provides a forum where risk assessors from different agencies have been able to share ideas on how to meet the requirements of the 2001 Information Quality Act.[2]
Filling Data Gaps. Risk assessment may be used as a tool to identify and prioritize research needs. In fact, perfect information would obviate the need for risk assessment. Data gaps result in uncertainty, to varying degrees, in the risk assessment results and predictions. How are data gaps addressed? The RAC provides a forum for discussing data needs. Research is the primary mission of some RAC member agencies. Others are granting agencies that fund and oversee research portfolios. These agencies and others work hard to assure that the data generated are applicable to the food safety problems of today. However, the enormity of our information needs still require outside research resources. The RAC workgroup on data gaps has summarized many of the research needs recognized by risk analysts for several key food safety risk assessments. The list of data gaps is posted on the Clearinghouse website, where they are available to all researchers. Efforts to publicize the data gaps are currently underway through articles like this one, as well as listservs and presentations at meetings.
Peer Review. The potential for risk assessments to influence risk management decisions and public health policy focuses a microscope on the accuracy and quality of any risk assessment. Peer review continues to be the traditional method of review and oversight, and agencies are taking another look at what constitutes an adequate peer review, who is qualified as a peer reviewer, and what types of peer reviews are necessary. For example, some risk assessment activities might require reviewers who look at every data source, validate every spreadsheet entry, and verify all of the associations in the computer model. Other risk assessments require review from specific subject matter experts who are intimately familiar with a particular process. Some risk assessments require both, or even review by an organization such as the National Academy of Sciences. Through a work group, the RAC has been actively involved in discussions on peer review and has co-sponsored, with the Society for Risk Analysis and JIFSAN, a symposium on what constitutes appropriate peer review for regulatory risk assessments. The RAC has also served as a means of getting initial peer reviews by other agencies, taking advantage of the fact that the member agencies employ some of the top world-wide recognized experts in risk assessment. These efforts have raised awareness among federal member agencies about the processes in other agencies, and the public’s expectations for review of government work products.
The RAC Needs You
The examples above only begin to describe the RAC’s involvement and influence in the continuous drive for more useful risk assessments. Among other things, the RAC promotes collaboration in the development of risk ranking models for food safety priority setting, helps build the link between microbiological criteria and public health goals, establishes recommendations to improve the utility of outbreak investigations, tests proposed frameworks against actual risk assessment experience, and expands the use of clinical and laboratory studies for estimating dose-response.
The RAC continues in its efforts to help improve food safety risk assessment, but cannot do it alone. It needs help filling data gaps, identifying data that can be hosted by the RAC, and learning from others about approaches to peer review, data quality, and many more issues. Our workshops and symposia allow opportunities to meet the analysts from many of the member agencies. The composition of the RAC’s current workgroups and agency representative contact information can be found on the Clearinghouse website. We encourage all interested parties to participate.
Marianne Miliotis, Ph.D., is as a risk assessment project manager in the Research and Risk Analysis Coordination Staff in the Office of Science at FDA’s Center for Food Safety and Applied Nutrition. Dr. Miliotis served as the lead for both the 2005 FDA Vibrio parahaemolyticus risk assessment and the CODEX Committee on Food Hygiene discussion paper on Vibrio spp. She is the current technical chair of the Interagency Risk Assessment Consortium.
Wesley R. Long, Ph.D., is the Risk Analysis and Extramural liaison in the FDA Center for Food Safety and Applied Nutrition’s Academic Liaison staff. Dr. Long was the founding chair of the Interagency Risk Assessment Consortium and co-PI on the JIFSAN Food Safety Risk Analysis Clearinghouse. He was also co-lead on the 2000 USDA-FDA Listeria monocytogenes Risk Assessment and is expert in food safety risk analysis issues across the federal government. He also co-administers the JIFSAN Professional Development Training Program in Food Safety Risk Analysis.
Series editor Sebastian Cianci is a policy analyst and a member of CFSAN’s Office of Food Safety Defense and Outreach. He serves as the Center’s trade press liaison.
References
1. Risk Assessment Consortium. foodrisk.org/ Risk_Assessment_Consortium.htm.
2. Guidelines for Ensuring and Maximizing the Quality, Objectivity, Utility and Integrity of Information Disseminated by Federal Agencies. whitehouse.gov/omb/fedreg/
final_information_quality_guidelines.html
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →