How FDA’s “Threshold of Regulation” Program Works
“Threshold of Regulation” refers to a Food and Drug Administration (FDA) program that saves resources for both the Agency and the food industry, as the Agency meets its crucial mission to protect public health. The program exempts substances that come into contact with foods—substances in packaging or food processing equipment, for example—from being listed as food additives if they migrate into foods at levels that result in no appreciable risk to human health.
The program, which is administered by the FDA’s Office of Food Additive Safety, allows these substances to undergo an abbreviated review process, avoiding the more administratively burdensome food-additive petition process. Abbreviated review allowed by the FDA’s Threshold of Regulation (TOR) policy determines whether the specific use of a food-contact substance meets criteria ensuring that the intended use would pose no more than a negligible health risk. The process does not alter the safety standard applied to these substances.
To the contrary, the abbreviated process removes administrative burdens that contribute little or nothing to safety assurance, freeing resources so that they may be applied to more pressing public health issues. The development of this approach and related analytical tools has, in all likelihood, increased the level of safety assurance overall because it permits FDA reviewers to efficiently apply the sum of their collected data and information to each safety decision.
To date, the FDA’s TOR policy has saved the Agency an estimated 100 to 150 staff years of labor. It has also fostered important information exchanges between the FDA and the food-packaging industry. In addition, the TOR process has spurred innovations in packaging markets that offer consumers more convenience.
This article explains how and why the TOR policy developed and how to submit a TOR request to the FDA.
History
In 1958, Congress amended the Federal Food, Drug, and Cosmetic Act (the Act) to require premarket approval or review of food additives prior to their inclusion in food. The amendment provided the legal framework for ensuring the safe use of food additives or chemicals in food by (1) precisely defining “food additive” and (2) establishing the standards of review and safety for food-additive approvals.
The Act defined “food additive” to include components of food-contact articles (food packaging and food processing equipment) that migrate, or may reasonably be expected to migrate, into food, making them subject to premarket approval [Section 201(s) of the Act]. For premarket approval for use of food additives, the Act required that the safety of the proposed use be demonstrated by data submitted to the FDA in a food-additive petition.
In the wake of these events, the FDA began to routinely receive requests for exemptions. Initially, the Agency issued informal opinions that allowed the use of specific substances that it did not believe invoked the food-additive provisions of the Act. During this time, industry also approached the FDA to propose that the Agency handle the issue of minimal migration of these substances into food through a so-called “Threshold of Regulation” policy.
A 1967 proposal suggested a 100 parts-per-billion (ppb) dietary concentration for food-packaging materials, excluding pesticides and heavy metals, based on the results of two-year chronic oral feeding studies of 220 compounds. In 1969, FDA Associate Commissioner L.L. Ramsey proposed a threshold of 50 ppb for levels of migrants into food or food-simulating solvents. Finally, in 1977, the Society of the Plastics Industry petitioned the FDA to modify its regulations so that uses of substances that resulted in levels of migration into foods at levels no higher than 50 ppb would be exempt from food-additive regulation unless they raised safety concerns.
Why did it take so long for the FDA to establish a formal policy? Legal and scientific hurdles kept the FDA from reaching this milestone until 1995. Until a court case clarified the issue in 1979, it had been unclear whether or not the FDA had authority to exempt additives from the formal petition process. In addition, until the 1980s, large, homogenous collections of toxicity data on which to base a threshold level were not available. The scientific analysis necessary to establish a dietary concentration level below which an abbreviated review would be protective could not even be performed until then.
In establishing a TOR, the FDA had to determine a level and establish a review framework that would meet the “reasonable certainty of no harm” standard, including the prohibition against FDA authorization of known carcinogens under the Delaney Clause [Section 409(c)(3)(A) of the Act]. The FDA had to choose a TOR low enough to ensure that the public health would be protected even if an exempted substance was later found to be a carcinogen.
In 1986, the FDA proposed a probabilistic approach to the TOR to address overall safety and concerns about potential carcinogenicity of untested additives. In 1987, scientists outside of the FDA considered factors that suggested a practical threshold between 1 part per trillion (ppt) and 5 ppb in the diet. In 1990, the Canadian Center for Toxicology proposed a dietary concentration of 1 ppb as the TOR for components of food-contact articles for which no toxicology data had been developed. The FDA’s policy was proposed in 1993 and finalized in 1995.[1]
The Threshold of Regulation Program
When should a TOR request be submitted? Substances used in food-contact articles (food packaging or food processing equipment) that migrate, or that may be expected to migrate, into food at negligible levels may be reviewed under the TOR process outlined in Title 21 of the Code of Federal Regulations (CFR) part 170.39, Threshold of Regulation for substances used in food-contact articles.
Submitting a TOR Request. Anyone may submit a TOR request to the FDA, and 21 CFR 170.39 outlines the criteria and process for submission.
How can you tell if your indirect food additive qualifies for exemption under the TOR process? A food-contact substance may be exempted from regulation as a food additive if the following general criteria apply:
• Migration of a substance is not expected to result in dietary concentrations above the threshold.
• The substance has not been shown to be a carcinogen in humans or animals, and there is no reason to suspect, based on its chemical structure or other criteria, that it is a carcinogen or a potent toxin.
• The substance, under its intended conditions of use, must not produce a technical effect in food into which it migrates (for example, an antioxidant that resided in the packaging would not have an antioxidant effect in the food into which it migrated).
• The substance use has no significant adverse impact on the environment.
To demonstrate that all the general criteria are met, submission of a data package is required, as outlined in Table 1 and in CFR 170.39. Generally, the data package does not have to be lengthy, and the request often can be submitted via a simple letter to the Agency, describing the substance and the details of how it is to be used. Submissions often can rely on information from product specification sheets or Material Safety Data Sheets, and submitters should consult with the Agency to avoid engaging in unnecessary testing.
Some examples of TOR submissions are for food-processing applications, polymerization-processing aids, colorants, alloys, and ceramics, and for cation or anion substitutions in chemicals. Typical TOR submissions involve uses in which there is little potential for migration into food, either because of the physical characteristics of the material or because the characteristics of the application or use make migration unlikely.
Examples include substances used in repeat-use, food-contact materials, such as conveyor belts, or components of food-processing machinery that may be in contact with large quantities of food during its lifetime. Such substances can be relatively insoluble compounds, such as inorganic colorants and materials like cross-linking agents used at relatively low levels in food-contact materials. Other examples include materials like ceramics that are highly resistant to corrosion and abrasion.

Figure 1 shows how a submitter can determine whether his or her food-contact substance (FCS) qualifies for TOR exemption.
The requestor should:
• Identify the FCS that is the subject of the request
• Estimate exposure
• Evaluate 21 CFR 170.39 criteria (that is, chemistry, toxicology, and environmental data requirements)
• Submit submission based on 21 CFR 170.39 criteria to FDA (four copies)
Review Process for a TOR Request. The TOR process is initiated when a TOR exemption request arrives at the FDA’s Office of Food Additive Safety and is assigned to a Consumer Safety Officer (CSO). The TOR submission is distributed to the TOR Committee for assignment. The review team consists of a CSO, a chemist, a toxicologist, and an environmental scientist. The four-person committee reviews the exemption request using a team approach, and summarizes its review in a joint memorandum (Figure 2).
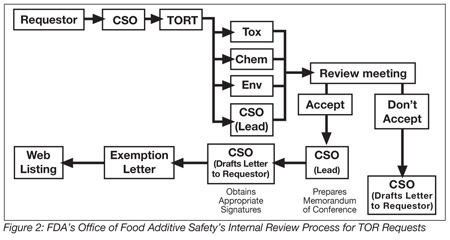
FDA Response. Usually within 60 to 90 days of receiving a TOR exemption request, the FDA will inform the request’s submitter, in writing, of the outcome of the review of the data package and whether the intended use of the food-contact substance qualifies for exemption under 21 CFR 170.39. It should be noted that, like food-additive regulations, exempted uses are generic. Therefore, any manufacturer/supplier can market an exempted substance, as long as the identity and intended use are the same as those for which the exemption was issued.
Find Out More
The TOR policy is a legally defensible, science-based exemption that results in significant savings in resources for both the FDA and the regulated industry while protecting public health. The FDA encourages individuals contemplating submission of a TOR request, or who are interested in knowing more about exemption under the TOR process, to review the Center for Food Safety and Applied Nutrition’s Guidance for Industry (www.cfsan.fda.gov/~dms/ torguid.html).
The Office of Food Additive Safety also lists exemptions that have been issued under 21 CFR 170.39. This list includes the name of the company that made the request, the chemical name of the substance, the specific use for which the substance received an exemption from regulation as a food additive, and any appropriate limitations on the substance’s use. As noted above, manufacturers and suppliers may wish to review this list of exemptions to determine whether an exempted substance they are interested in marketing is currently listed for the same proposed, intended use. Questions about this list should be directed to the Office of Food Additive Safety (premarkt@cfsan.fda.gov).
Anna P. Shanklin, Ph.D., is a chemist and consumer safety officer in the Division of Food Contact Notifications of the Office of Food Additive Safety, in FDA’s Center for Food Safety and Applied Nutrition (CFSAN). She has been with CFSAN for eight years.
Susan Cahill is a writer/editor in FDA’s Center for Food Safety and Applied Nutrition, in the Office of Food Defense and Center for Emergency Response.
References
1. More detailed information about FDA’s TOR policy is available in the proposed rule published in the Federal Register on October 12, 1993 and the Final Rule published in the Federal Register on July 19, 1995.
Resources
Flamm, W.G., L. R. Lake, R. J. Lorentzen, A. M. Rulis, P. S. Schwartz, and T. C. Troxell. 1987. Carcinogenic potencies and establishment of a threshold of regulation for food contact substances, in De Minimis Risk, Vol. 2 of Contemporary Issues in Risk Assessment. Edited by D. Whipple. New York: Plenum Press.
Food and Drug Administration. Food Additives: Threshold of regulation of substances used in food-contact articles: Proposed Rule. Federal Register 58:52719-52729 (1993).
Food and Drug Administration. Food Additives: Threshold of regulation of substances used in food-contact articles: Final Rule. Federal Register 60:36582-36596 (1995).
Frawley, J. P. 1967. Scientific evidence and common sense as a basis for food-packaging regulations. Food. Cosmet. Toxicol. 5:293-30.
Munro, I. C. 1990. Safety assessment procedures for indirect food additives: an overview. Regulatory Toxicology and Pharmacology 12:2-12.
Ramsey, L. L. 1969. The food additive problem of plastics used in food packaging. Presented at the National Technical Conference of the Society of Plastics Engineers. Commonly referred to as the Ramsey Proposal.
Rulis, A. “De Minimis and the Threshold of Regulation.” In Food Protection Technology, edited by C. W. Felix, 29-37. Chelsea, MI: Lewis, 1986.
Society of the Plastics Industry, 1977. Citizen petition submitted to FDA to amend the food additive definition in 21 CFR 170.3(e), Docket No. 77-0122, March 30, 1977.
>
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →







