Comparison of Microbiology Testing Practices
Microbiology testing practices at food plants around the world vary extensively. As a result, food safety officers need to understand those variations and establish expectations and practices with their suppliers to mitigate variations and ensure safe and wholesome products that meet label claims.
Microbiology testing in food plants around the world varies by organism, food segment (protein, dairy, fruit/vegetable, processed food) and geographic region. This article will examine variations in global testing practices in more detail, including a review of where samples are collected and the test methods used for analysis. It will not cover variations such as where and how food samples are analyzed (i.e., type of lab, procedures and staff) and differences in testing based on plant size and ownership (i.e., single-plant vs. part of a global food organization).
The data and charts in this article come from the new market report from Strategic Consulting Inc. (SCI).[1] Food Micro, Eighth Edition: Microbiology Testing in the Global Food Industry is drawn from primary research interviews with QA/QC managers in more than 450 food plants in 19 countries, including China and India.
Worldwide Food Microbiology Testing
In general, global test volumes are increasing and are up 128 percent over the past 15 years. Pathogen testing is growing at an even faster rate and represents an increasing percentage of total food microbiological testing. Fifteen years ago, pathogen testing represented 13.7 percent of total micro tests. In 2013, it represents 23.2 percent of all tests (Figure 1).
The world’s food chain is increasingly complex, with food shipments across borders growing very rapidly. In the U.S., for example, imported food now represents 15–20 percent of all food consumed. According to the U.S. Department of Agriculture, total imports have increased 7 percent per year since 1999. In the past 10 years, imports of animal-based foods have increased by 5 percent and plant-based foods have grown more than 8 percent.
On the surface, these two trends seem to be in concert—the global food chain is becoming more important and food micro testing is increasing. A closer look, however, reveals deeper inconsistencies that are concerning.
Geographic Differences
The general increase in food microbiological testing as well as the more dramatic increase in pathogen testing is not consistent across the geographic regions of North America (NA), Europe (EU), Asia or other regions of the world (ROW). In North America, pathogen testing has grown at greater than 10 percent for the past few years, whereas in the EU, it has grown at half that rate.
In general, SCI research shows that each geographic region has different trends/drivers affecting growth, and these factors combined with public perceptions about food safety influence testing practices in the region. Clearly, it’s important to consider the geographic source of food imports, as well as the food segment, to determine further testing requirements that should be added to the process.
Microbiological testing by food segment (protein, dairy, fruit/vegetable, processed food) also varies around the globe. The protein segment, which includes beef, pork, chicken, fish and eggs, represents 27 percent of overall microbiological testing in the food industry but more than 40 percent of total pathogen testing. The dairy segment (fluid milk, cheese and other dairy-based products) represents 23 percent of total testing but just 10 percent of pathogen testing.
As any examination of foodborne illnesses demonstrates, problems can—and do—occur in any of the food segments. Over the past 20 years, the protein segment has received the majority of publicity, and the regulations and pathogen testing levels reflect that fact. The low levels of testing in some of the other food segments might not make sense given the increasing foodborne illness events over the past few years.
Where food samples are collected is another area of significant geographic variation. Worldwide, 26 percent of all food microbiology samples are collected from raw materials, and 25 percent are collected in-process and in-plant/environmental. The remaining 49 percent of tests are collected from end products prior to release. Sample collection within geographic regions, and when looking only at pathogen tests, shows major differences (Figure 2).

In North America, just 8 percent of pathogen samples are collected from raw materials, whereas in-process/environmental sampling is much more aggressive at 44 percent. In contrast, just 8 percent of pathogen samples in Asia are collected from in-process/environmental sources, indicating that very different testing philosophies are at work. While Hazard Analysis and Critical Control Points plans (and other programs) have been at the heart of food safety programs in North America for the past 20 years, it appears this is not yet the case in China, India and other Asian countries interviewed.
The Campylobacter Example
An examination of one pathogen, Campylobacter, will illustrate testing variations more thoroughly. A recent U.S. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report (April 19, 2013) summarized the 1996–2012 trends in illness from pathogens commonly transmitted through food. The report showed that after years of substantial declines, Campylobacter infections had increased to their highest level since 2000, representing one of the leading causes of bacterial foodborne illness for the period.
Yet global test volume for Campylobacter in 2013 is estimated at 4.6 million tests—just 2 percent of total pathogen testing worldwide. In addition, only 14 percent of food plants interviewed by SCI indicated they test for Campylobacter. Not many plants test for this pathogen, and when they do, it is at a much lower rate than for other organisms.
A closer look at the 14 percent of food plants testing for Campylobacter shows variations both by food segment and geographic region. In North America, 25 percent of protein plants test for Campylobacter, but no other food segment tests for this pathogen. In Asia, however, every food segment but fruit/vegetable tests for the organism (Figure 3).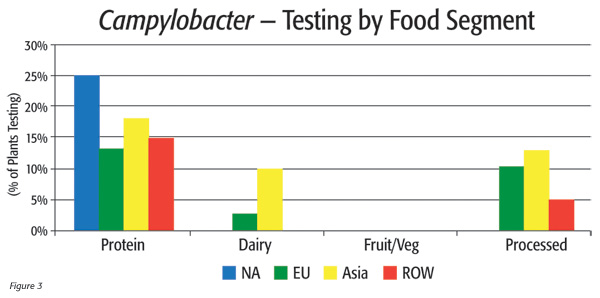
Across those plants testing for Campylobacter, the test methods vary regionally. In total, 76 percent of Campylobacter tests worldwide are performed using traditional culture methods, with the next leading analytical method, antibody-based tests, representing just 12 percent of test volume.
On a geographic basis, Asian plants use only traditional methods for Campylobacter analysis, and in the EU, only traditional and antibody-based methods are used. In North America, the majority of analysis is done with newer, rapid methods. The regional variation in test methods seen for Campylobacter is like that seen with other pathogen test methods. Different methods can result in different food safety decisions since method-to-method equivalency is not always perfect (Figure 4).

In spite of the increasing incidence of foodborne illness from Campylobacter, it appears that minimal pathogen-specific testing is done for the organism. Even when Campylobacter testing is done, there are considerable differences among the geographic regions, food segments and where samples are collected.
If we were to draw one overall conclusion about global food pathogen testing practices, it would be that food testing worldwide is far from homo-geneous. There are important variations by organism, by food segment, by geographic region, by where samples are collected and by the method used for analysis.
Given these inconsistencies, how do we consistently keep our food safe? The best way to start is to acknowledge and understand the variances and then use that knowledge to specify and require that all inputs to your product be monitored using a well-defined, consistent and clearly documented approach. As we know, problems can occur even at factories utilizing optimum food testing programs. Given this, it is imperative to minimize the risks associated with the testing variances that exist globally.

Reference
1. Strategic Consulting Inc., 2013. Food Micro, Eighth Edition: Microbiology Testing in the Global Food Industry. www.strategic-consult.com. >
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →