Inhibiting Listeria Growth to Improve Food Safety
An earlier installment of this column by my good friend John Butts—“The Journey to a State of Control”—described the last decade of effort by the processed meat industry to reduce pathogen contamination risks in pre-packaged, ready-to-eat (RTE) products. John provided an excellent historical context for how a highly competitive industry came together to address previously unrecognized food safety issues that our continuously developing society uncovers. This attitude of supporting research to continuously improve reflects high business ethical standards. No one has any desire to see the products they produce cause any harm to their customers.
I would like to build on John’s discussion by bringing in a few other approaches in addition to the environmental controls and interventions that were discussed. By the late 1990s, risks of contamination by Listeria monocytogenes in processed meats were better understood, leading to its classification as a zero-tolerance pathogen. However, risk analysis conducted by the U.S. Food and Drug Administration (FDA) and the U.S. Department of Agriculture (USDA) showed that human illness required exposure to fairly high numbers of organisms. Thus, in addition to preventing the organism from getting into products, researchers thought additional strategies to limit growth could be effective in improving food safety, although not necessarily satisfying zero-tolerance regulatory requirements.
Risk Reduction Approaches
Minimizing microbiological risk has long employed the hurdle concept that was originally proposed by Leistner and co-workers at the German Meat Research Institute in the 1970s. Simply put, multiple hurdles address the fact that no single step or intervention will ever be perfect in eliminating a risk from a pathogen. We humans are just not smart enough to anticipate every contingency, or good enough to execute a single food safety plan without any errors. Instead, multiple hurdles in series or parallel will combine to achieve desired levels of safety. Therefore, with respect to L. monocytogenes, we can think of a number of hurdles to address this pathogen. We know that we adequately kill any Listeria organism that may exist on our raw material with cooking processes. We have decreased the likelihood of recontamination after cooking by at least a factor of 50 in the last 10 years through sanitary equipment design, better cleaning and sanitizing procedures and “seek and destroy” environmental monitoring programs. These hurdles keep the organism out of the finished product. Many companies go beyond this step to add additional hurdles to either treat the product in the package to inactivate any pathogens that made it past the original hurdles or to add ingredients that will inhibit their growth.
Post-package approaches have a long history of use in food processing. Canning was invented in 1810 by Nicolas Appert to prevent spoilage (and toxic botulinal growth) in foods used by Napoleon’s army. The more mild thermal treatment of pasteurization was developed in the late 1800s and is effective against vegetative microbes like Salmonella, Listeria and lactic acid spoilage bacteria. Some consider these principles as enabling the development of large-scale food processing that has evolved over the past 200 years. Modern refinements have used computing power and time/temperature lethality relationships described in the Ball equation to verify that thermal processes deliver safe RTE foods for consumers. The last few decades have also seen application of ultra high-pressure treatment or exposure of packaged products to ionizing radiation from gamma- or beta-ray sources to achieve the same effects.
Two other approaches have been historically used to maintain safety of food during distribution and storage. Drying to reduce moisture content to a point where bacteria cannot grow was once a primary approach, but today, with the advent of refrigeration and anaerobic packaging, it is much less common. Salting or the introduction of ingredients or additives that prevent or retard bacterial growth also have a long history of use. Sodium nitrite as a curing agent in meat processing plays an important role in botulinal and other pathogen protection. Salt and nitrite as two basic ingredients were considered sufficient for food safety until 25–30 years ago when we began to recognize that L. monocytogenes infections had, among other causes, a processed meat and poultry transmission vector. Thus, in the late 1990s and early 2000s, a search was on at universities and industry research and development groups for other ingredients that could be of use. The first wide-scale approach involved addition of sodium or potassium lactate in combination with sodium diacetate to cured RTE products.
Sodium and potassium lactate salts were originally introduced to the meat and poultry industry in the 1980s when processed turkey products became much more popular. This coincided with the development of cook-in-the-bag packaging and processing advances for large-sized products used in foodservice. Since poultry products were traditionally uncured (i.e., made without nitrite) and had much lower salt levels than their red meat counterparts, many in the industry had a concern around the risk of botulinal growth under temperature abuse. The lactate salts provided an additional protective barrier or hurdle especially for uncured, cook-in-the-bag products. They had lesser use in sliced or repackaged uncured products because the contaminating bacteria introduced by such handling were felt to provide a similar food safety measure by spoiling the product before pathogens could grow. The concept of using bacterial spoilage of a product as a food safety measure is no longer considered a reliable approach.
Lactate salts by themselves will inhibit the growth of Listeria in processed meats; however, unreasonably large amounts are required. However, in combination with sodium diacetate, realistic levels can be used and a balance achieved between growth inhibition and product quality. Sodium diacetate is an interesting ingredient. Chemically, it can be considered a half-neutralized acetic acid (often derived from vinegar) or a complex of a molecule of acetic acid and a molecule of sodium acetate. The beauty of both lactate and diacetate is that they are metabolites in human energy metabolism, so chemical safety concerns about them as ingredients are minimal. Meat also has a background level of lactate between 0.5 and 1%, arising from the postmortem metabolism of glycogen stores in muscle.
The action of lactate and diacetate is to interfere with bacterial metabolism, thus inhibiting growth. Since 2004, the use of these ingredients has become an important added food safety hurdle, and over half of the cured RTE processed meats in the U.S. utilize these ingredients today. There have been over 100 publications from various laboratories confirming their effectiveness under a variety of conditions. The growth inhibition even extends into aerobic storage of products, which is important for products sliced in a deli or those inadvertently contaminated during consumer handling.
Effects on Foodborne Illness
It is noteworthy to look at the effects of inhibiting growth on the likelihood of illness. FDA and USDA released an exhaustive risk analysis for foodborne listeriosis in 2003. In that report, the quantitative exposure to L. monocytogenes versus the probability of illness and mortality was estimated for three groups of humans: unborn children; normal; and elderly, using the best available data at the time. We still need more data to improve precision, but some general principles are known and we can apply calculations of exposure scenarios from that report. Let’s start with a hypothetical RTE product inadvertently recontaminated with only 10 organisms per gram. Unchecked Listeria growth during refrigerated storage will result in a product with levels up to log 8–9 colony-forming units per gram (i.e., 100 million–1 billion organisms per gram) within 3 to 4 weeks. Then, if a pregnant woman were to consume two ounces of the product at that point, her risk of a miscarriage could be around one in 7,500. This is a serious risk. If the same product is formulated to inhibit Listeria growth to 1 or 2 logs, that is, the numbers of Listeria would be limited to 100 to 1,000 per gram, then the risk profile decreases to between one in 75 million and one in 50 billion. It is this significant risk reduction that led many companies to add lactate and diacetate to their formulations (Figure 1).
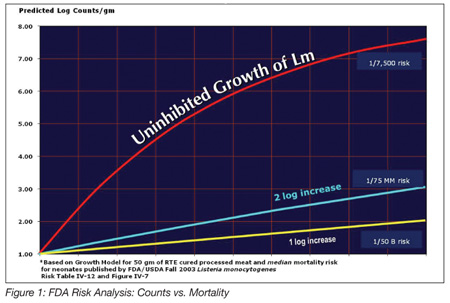
Another reason for the widespread use of growth-inhibiting ingredients was the USDA rule issued on an interim basis in late 2003 and finalized in 2005. It specified three regulatory options to address L. monocytogenes risk for RTE meat and poultry products. These can be found in 9CFR430.4. The options acknowledge the multiple hurdle mentality, but the regulatory numbering system is the opposite of the number of Listeria hurdles. An Alternative 3 system relies on environmental controls to prevent recontamination after processing. Alternative 2 adds either a growth inhibitor ingredient in the RTE product or a post-packaging treatment that is validated to reduce the number of Listeria. Alternative 1 represents the use of both a growth inhibitor and a post-package treatment. The regulations also describe regulatory consequences of finding Listeria on food contact surfaces in the processing facility with differences for each alternative with respect to finished product sampling. Alternative 1 and 2 systems offer manufacturers a little more regulatory flexibility because they have the safety extra hurdles in place.
Since the development of lactate and diacetate to inhibit Listeria growth was commercialized, there have been many other ingredients introduced that have enlarged the array of choices for processors. These include blends of organic acid salts and other natural fermentation products, bacteriocins, phages, some natural antioxidants and other ingredients. A list of approved substances with their labeling requirements is found in USDA – Food Safety and Inspection Service’s (FSIS) directive 7120.1, which can be accessed at www.fsis.usda.gov/Regulations_&_Policies/7000_Series-Processed_
Products/index.asp.
How effective have all these approaches been? Before FSIS published the proposed rule, the FDA and Centers ?for Disease Control and Prevention (CDC) estimated that each year L. monocytogenes caused over 2,500 cases of foodborne illness, including 500 fatalities in the U.S. FSIS estimated that about two-thirds of these cases were attributable to RTE meat and poultry products. In January 2011, the CDC published revised estimates of all foodborne illness in the U.S. While they have cautioned about comparisons with the prior estimates due to methodology changes, it is reassuring to note that listeriosis cases and deaths are now estimated to be significantly lower with a mean of 1,600 cases and 255 deaths. The decline in yearly laboratory-confirmed Listeria infections has been more modest at 25–30% for 2006–2009 compared to a 1996 baseline period. Additionally, monitoring data by FSIS of Listeria prevalence in RTE processed meat and poultry showed a marked decline between 1998 and 2009.
As encouraging as these results are, we still have not achieved the federal government’s Healthy People 2010 goal of fewer than 0.25 cases of listeriosis per 100,000 individuals. Furthermore, the 2020 goal has been set at 0.20. We need to address the risk from Listeria contamination of products in store delis and sandwich shops. These are not traditional federally or state-inspected locations, and hence, there has not been the intensity or focus to address the Listeria risk in those locations compared to centralized manufacturing facilities. Yet, a large amount of RTE meat products passes through those channels.
One thing we have learned about food safety is that if we stand still, we will regress. Further improvements on our impressive record will need development of more hurdles to Listeria contamination, survival and growth in processed meat products.
Andrew L. Milkowski, Ph.D., is an adjunct professor at the University of Wisconsin-Madison. During a career with Oscar Mayer, he was responsible for food safety research and development. He is a member of the Institute of Food Technologists and the American Meat Science Association. >
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →








