From Apples to Poultry: How to Organize Your Hazard Analysis
Clearly identifying and categorizing potential hazards is key to producing a useful hazard analysis—no matter if you are processing apples, yogurt, frozen enchiladas or ready-to-eat chicken. It is common to identify biological hazards one-dimensionally by either naming the organism directly, such as “Salmonella” and “Listeria,” or by simply categorizing them as pathogens, vegetative pathogens, or spore-formers. Sometimes, but not always, categorizing these hazards according to their origin and into groups of similar organisms can be quite useful in dealing with them in the hazard analysis. However, that is only part of the task.
The purpose of the hazard analysis is to identify potential hazards, evaluate them in terms of their likelihood of occurrence, and to identify control measures when needed. To get to the establishment of effective control measures at critical control points (CCPs), the hazard analysis must not only be based on complete information, but it must be properly organized for use. For example, classifying biological hazards according to origin and microbiological categories suggests the control measures necessary to prevent the occurrence of hazards. The hazard analysis begins with identifying hazards at each ingredient or processing step in the process. It asks which potential food safety hazards are introduced, controlled, enhanced, or, one might even say, “associated” with this step. The conditions that are associated with the occurrence of these hazards are informative in terms of their reduction, avoidance and control (Table 1). Let’s look at some examples and review how the use of a simply formatted Hazard Analysis Worksheet can help increase the effectiveness of any operation’s hazard analysis process.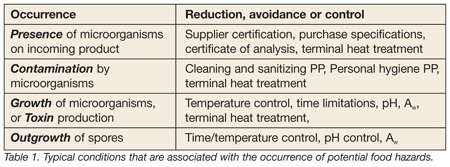
A Core Examples
The first step in hazard analysis is to try to group microorganisms by common characteristics. Instead of identifying E. coli O157:H7, Salmonella, Campylobacter and Listeria, you may want to group them into “vegetative pathogens.” This grouping tells us that a terminal heat treatment using pasteurization conditions is a valid control measure. If the grouping was “spore-formers” that would not be the case. What about grouping toxin producers together? This would tell us that control of pH would be an effective control measure to prevent outgrowth of spores. It would indicate that processing at higher temperatures to conditions of commercial sterility might be necessary to destroy the spores. This grouping of hazards might also suggest the critical limits at the CCP.
Now let’s drill down and look at the potential hazards for a specific food—a load of incoming apples from a refrigerated storage facility—and categorize the hazards to see how this works. The apples have been in cold storage for six months and are going to be cored, peeled and sliced in order to be used to be used in apple pie filling. They are dumped into a flume of water to cushion them against bruising. The water is recycled and retreated in a countercurrent flow. The apples are passed over a scrubber that removes considerable foreign material and exposes rot. They are rinsed and passed over a conveyor for visual inspection, culling and trimming before proceeding to the peeling and slicing operation. We can look over the receiving part of the process and complete a hazard analysis to illustrate what we have been discussing.
Since apples are received from a refrigerated storage facility, they can be assumed to be contaminated with common pathogens from the soil and the environment, including those from pests. Yeasts and molds will be present in addition to the bacteria. We can identify vegetative pathogens and spore-forming pathogens with the help of the hazard guide that the U.S. Food and Drug Administration (FDA) has produced for juice (www.cfsan.fda.gov/~dms/juicgu10.html). Potential hazards are:
B1. Presence of spore-forming and vegetative pathogens
B2. Contamination with sporeforming and vegetative pathogens from handling equipment
B3. Contamination with sporeforming and vegetative pathogens from reused water in flume
C1. Presence of pesticides on incoming product
C2. Presence of patulin in rotten apples
P1. Presence of rocks and sticks on incoming product
In considering each of these individually, we can clarify the considerations that should be taken into account when conducting the hazard analysis.
B1. Presence of spore-forming and vegetative pathogens. For the first biological hazard, the presence of spore-forming and vegetative pathogens cannot be avoided. One of these pathogens is Cryptosporidia, which will survive the minimum pasteurization process but not the cooking and the hot fill at the 218F required for processing. Proper storage conditions, supplier’s certifications and specifications might reduce the hazards to a lower level but there is no avoidance of the potential hazard so these are still judged to be likely to occur. Since they are likely to occur, a control measure of cooking to at least 165F for 3 seconds must be identified and applied at a CCP.
B2. Contamination with sporeforming and vegetative pathogens from handling equipment. The likelihood of contamination with spore-forming and vegetative pathogens can be reduced effectively by a properly implemented and effective sanitation program. The potential hazard will be judged to be not likely to occur and the justification will be the sanitation standard operating procedure (SSOP) #2, Condition and Cleanliness of Food Contact Surfaces.
B3. Contamination with sporeforming and vegetative pathogens from reused water in flume. The contamination with sporeforming and vegetative pathogens from the reused water in the transport flume is also unlikely to occur due to proper chlorination and rechlorination of process waters. This is effective against vegetative cells and spores. Process water chlorination, then, is part of SSOP #1, Safety of Water, and can justify the determination of the hazard analysis notation “not likely to occur.”
C1. Presence of pesticides on incoming product. Presence of pesticides on incoming product is minimized once the apples are washed and peeled. Since these unit operations are not controls that must be monitored to specific limit, these operations can be used to justify that the potential hazard is not likely to occur.
C2. Presence of patulin in rotten apples. Patulin is a mycotoxin that is produced by fungi commonly found on apples. High levels of patulin can be produced in rotting or moldy apples. Fallen fruit, apples that have been damaged (e.g., by insects or birds, or bruised, such as during handling) are more susceptible to the growth of patulin-producing molds. Storage of apples under conditions that are not inhibitory to the growth of molds also can lead to high levels of patulin in the apples. If fallen fruit, moldy, rotten, bruised or damaged apples, or improperly stored apples, are used to make juice, high levels of patulin may occur in the juice, including pasteurized juice, because thermal processing does not destroy it.
Patulin presence on raw apples is likely to exceed the legal limits established. However, this is not likely to pose a safety concern except in apple juice. The following citation from FDA states that “given the nature of the food, the manufacturing processes, or consumption practices for many foods, patulin does not appear to pose a safety concern, with the exception of apple juice. For instance, the rotten portions of most fruits and grains are typically removed prior to consumption.”
P1. Presence of rocks and sticks on incoming product. Presence of rocks, sticks and other foreign material on incoming product is removed by the washing, rinsing and visual inspection processes. The requirement in the Current Good Manufacturing Practices (cGMP) states, “Effective measures shall be taken to protect against the inclusion of metal or other extraneous material in food. Compliance with this requirement may be accomplished by using sieves, traps, magnets, electronic metal detectors, or other suitable effective means.” The unit processes that separate foreign materials are adequate justification that potential hazards are not likely to occur.
We can now fill out a Hazard Analysis Worksheet from these notes (Table 2). These will be different for each facility and process. Remember to fully identify the hazards and classify them according to their occurrence and group them in order to make avoidance procedures or control measures obvious and to prevent duplication of effort.
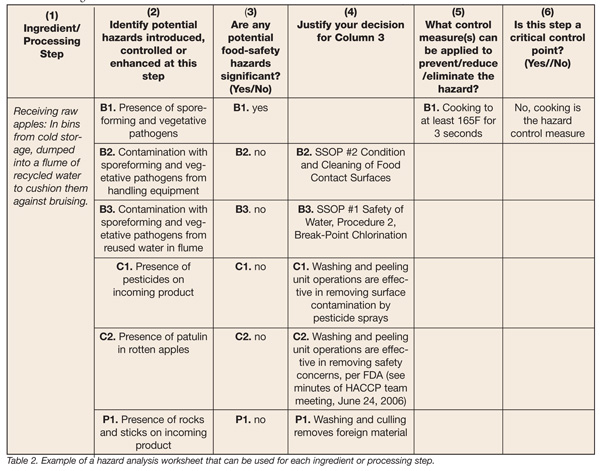
The worksheet is elegant in its simplicity, including just six columns of information to be provided or questions answered, and can be easily developed for use in any operation. The first column provides space in which to list the specific ingredient or processing step for which you are conducting the hazard analysis. In our example, we’ve used the receipt of the raw apples and described the basic receiving process as shown in Table 2. The next column of information to be provided is to identify potential hazards introduced, controlled or enhanced at this step in the process, which includes the six hazards we’ve detailed above, from the presence of spore-forming and vegetative pathogens and the presence of rocks and sticks on incoming product. For each of these six, we want to answer the Yes/No question in the third column: Are any potential food-safety hazards significant? In other words, are they able to occur? In Column 4, the analysis will justify the answer to the Column 3 query. As shown in the table, for example, we are able to provide justification that hazards do not exist or are not likely to occur in the operation’s apple receiving processes for the identified hazards B2, B3, C1, C2 and P1. For B1, we leave the space blank to indicate that this has been identified as a food safety hazard likely to occur for this processing step, and continue on to Column 5’s question: What control measure(s) can be applied to prevent, reduce or eliminate the hazard? In the case of the hazard posed by the presence of spore-forming and vegetative pathogens (B1), we know that cooking the apples to at least 165F for 3 seconds will significantly reduce, if not eliminate, the hazard, and we complete the form accordingly. At this point, we can complete the hazard analysis by deciding whether this step is a CCP. Since cooking is a proven control measure, we can indicate “No” on the form.
A Poultry Pop Quiz
Let’s try an exercise. We will use as an example raw chicken breasts that arrive fresh to be marinated and breaded, then baked and packaged. Since the resulting product is fully cooked, the foodservice operation just has to “re-thermalize” the product and serve it. Raw boneless chicken breasts are received refrigerated and immediately passed through a brine injector which injects a solution of water, salt and sodium phosphate through injector needles. The breasts are coated with a breading consisting of a base of water and hydrocolloid gums, and then are dusted with a breading of wheat flour and dry spices and herbs. A conveyor takes the breasts into the oven, then through a cooling tunnel and into a spiral freezer.
The U.S. Department of Agriculture’s (USDA) hazard guide lists the microbial hazards for raw poultry as primarily Salmonella and Campylobacter. The HACCP committee has identified the following hazards, based on the USDA guidelines:
B1. Presence of vegetative pathogens on incoming chicken breasts
B2. Contamination of chicken with vegetative pathogens and spore-formers from unclean equipment
B3. Contamination of brine solution with vegetative pathogens from chicken
B4. Contamination of breading and breading equipment with vegetative pathogens from chicken
B5. Growth of vegetative pathogens in chicken
B6. Growth of vegetative pathogens in brine
B7. Growth of vegetative pathogens in breading and on equipment
C1. Presence of a major allergen, wheat, in the breading, which is a concern if it is not declared on the label
P1. Presence of bones in incoming breast fillets
P2. Contamination of the chicken breast by pieces of broken needles from the brine injector
The following information has been collected on each of these hazards:
B1. Incoming chicken breasts are likely to contain Campylobacter and Salmonella. Cooking to temperatures of least 165F for 15 seconds will destroy these vegetative pathogens. The chicken breasts are cooked to a center temperature of 172F for 2 minutes.
B2. SSOP #2, Condition and Cleanliness of Equipment, is implemented, checked, corrected when necessary, and documented. It establishes procedures for cleaning equipment at the end of each shift and for intermediate cleanup at the end of each four hours of operation. GMP #3, Temperature of Processing Room, is in place and automatically recorded. Temperatures are maintained below 50F during processing.
B3. The brine solution is likely to become contaminated with vegetative pathogens from chicken. To minimize this, the injector is pumped out, washed and sanitized at each shutdown for a break and remaining solution is discarded. This procedure is found in SSOP #3, Prevention of Cross Contamination, Procedure 2, Minimizing Cross Contamination in Brining. However, even with these intermediate controls in place, pathogens are likely to be injected into the chicken breast if only from the surface of the chicken. The only control measure is the cooking process. Cooking to temperatures of least 165F for 15 seconds will destroy these vegetative pathogens. The chicken breasts are cooked to a center temperature of 172F for 2 minutes.
B4. The breading is recycled from the cascade breader and is reworked with fresh makeup as needed for a four-hour shift. At the break all used breading is discarded and the equipment is cleaned. This procedure is found in SSOP #3, Prevention of Cross Contamination, Procedure 3, Minimizing Cross Contamination in Breading. Again, even with these intermediate controls in place, pathogens are likely to be added to the surface of the chicken during breading. The only control measure is the cooking process. Cooking to temperatures of least 165F for 15 seconds will destroy these vegetative pathogens. The chicken breasts are cooked to a center temperature of 172F for 2 minutes.
B5. Growth of vegetative pathogens in the chicken is minimal because of the temperature and handling of the chicken breasts. Chicken breasts are received chilled and handled in a refrigerated room. One pallet of chicken is processed per hour and each pallet is emptied before the next pallet is used. GMP #3, Temperature of Processing Room, is properly in place and automatically recorded. Temperatures are maintained below 50F during processing. Campylobacter does not grow at temperatures below 80F and the growth of Salmonella is severely curtailed. Time is also a factor and the Food Code allows 4 hours at temperatures below 70F.
B6. Vegetative pathogens are unlikely to grow in the brine because the temperature is maintained below 50F. The brine injector is washed and fresh brine is added each 2 hours.
B7. Vegetative pathogens are unlikely to grow in the breading because the temperature is maintained below 50F. The breader is washed, used breading is discarded, and fresh breading is added each four hours.
C1. Wheat is listed by the FDA as one of the foods that is a major allergen. Wheat gluten can be a hazard to someone who is allergic. These persons are aware of their condition and avoid these products when the allergen is clearly listed in the ingredient statement on the label. When new labels are ordered, Quality Control is required to sign off on allergen labeling. SSOP #5, Protection from Adulteration, is in place and Procedure 9, Control and Labeling of Allergenic Ingredients, is in place.
P1. Sometimes chicken breast filets can have bone fragments in them. We have in place a specification for “Skinless and Boneless Chicken Breast Filets.” We also get a supplier’s guarantee that the filets have been examined through X-ray detection to detect any bones.
P2. Broken needles from the brine injector are not unusual. There are prerequisite programs in place, such as SSOP #5, Protection from Adulteration, Procedure 6, Controlling Metal Contamination, which include inspection of needles at the breaks and a metal detection step toward the end of the process.
With this information, we can now complete the six-column Hazard Analysis Worksheet for the poultry processing operation. You should be able to easily identify the CCPs, if any, with a high level of confidence that the hazard analysis has effectively addressed all potential bumps in the road to safe food production.
John E. Rushing, Ph.D., is Professor of Food Science, North Carolina State University and Department Extension Leader, NC Cooperative Extension Service. He works with the dairy and foods processing industries in North Carolina to improve the safety, quality and value of foods. He also provides training, technical assistance and guidance to extension agents, regulatory and public health agencies, and food entrepreneurs. As the Executive Director of the Southeastern Food Processors Association, he works to provide education and interchange of ideas between processors in the region. >
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →







.webp?t=1721343192)