Comparison of Filtration Units for Assessing Microbiological Recovery

In 2015, the U.S. Food and Drug Administration (FDA) finalized and enacted the Food Safety Modernization Act (FSMA), requiring food and beverage manufacturers to evaluate potential hazards and enact measures to prevent contamination.[1] Under the act, companies will be required to keep detailed records and establish written food safety plans. High-risk producers will now be subject to official FDA inspections once every 3 years versus once every 10 years under previous standards. [2] The regulations are designed to shift the food safety system from reactive to proactive when handling contamination. The legislation, the largest change in food safety since 1938, is seen as a shift away from internal quality control by food and beverage manufacturers to a system of federal oversight similar to the regulation applied to the pharmaceutical sector.
As a result of FSMA and other initiatives around the world, food and beverage manufacturers are intensifying their focus on prevention of contamination. An important part of these efforts is to ensure efficient and effective methods of bioburden testing, an essential part of preventing contamination and protecting consumer health.
Bioburden Testing of Liquids
In evaluating potential contaminants, devices and processes used to test liquids and water are critically important. Water in particular can be a major source of contamination, carrying potentially fatal Gram-negative bacteria. One variation, Clostridium perfringens, is responsible for sickening an estimated 1 million people each year in the U.S.[3]
Traditionally, microbiological testing of water and other liquids require processes that are time, labor and space intensive. As a result, the common “most probable number” technique of detecting and culturing contaminants has been replaced in laboratories with more efficient membrane filtration processes. Membrane filtration technology detects contamination or colony forming units (CFUs) by passing water or other liquids through a sterile membrane filter with a pore size small enough to retain bacterial cells (typically 0.45 µm). This process provides faster results, requires less space and is capable of processing larger volumes of liquids.[4]
Membrane filtration methods have become the standard for bioburden testing of liquids. Membrane filtration devices can be used with a variety of liquid samples including water, raw materials, in-process samples and final products. These devices minimize sample residue and allow the full volume of the sample to be more effectively tested for microbiological recovery, an essential step in the process of bioburden testing.
Microbiological recovery is necessary to confirm the nature of contamination and determine best methods for eradication. Once the sample is filtered, a growth medium is added to cultivate and evaluate recovery following federal standards such as the FDA Bacteriological Analytical Manual (BAM). In the U.S., specific validation methods and acceptable ranges of countable CFUs are mandated by the FDA BAM or internal standards of practice.
The faster the rate of microbiological recovery, the more efficient the filtration device. A number of factors can impact microbiological recovery including organism recovered, growth medium and incubation conditions.[5] We recently conducted a study comparing membrane filtration units and their ability to ensure optimal microbial recovery. While the study was conducted under strict pharmaceutical standards—U.S. Pharmacopeia—the results are equally relevant to the food and beverage industry, especially in the light of increased federal oversight. The results of this study indicate that recovery can be affected by membrane composition, raw material sources and sterilization processes. These findings indicate that membrane filtration systems can be reliable devices for bioburden testing, but should be selected carefully based on application and need.
Comparison of Filtration Units
 In this study, we compared EZ-Fit™ filtration units (EMD Millipore) to four other membrane filtration devices to determine recovery rates. The study utilized tryptic soy agar (TSA), Sabouraud dextrose agar (SDA) and a low nutrient agar (R2A), all culture media commonly used in regulation-required testing of raw and in-process materials and final products (Table 1). Similarly, we used five microorganisms also commonly seen in pharmaceutical and food/beverage testing: Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans and Aspergillus brasiliensis (Table 2).
In this study, we compared EZ-Fit™ filtration units (EMD Millipore) to four other membrane filtration devices to determine recovery rates. The study utilized tryptic soy agar (TSA), Sabouraud dextrose agar (SDA) and a low nutrient agar (R2A), all culture media commonly used in regulation-required testing of raw and in-process materials and final products (Table 1). Similarly, we used five microorganisms also commonly seen in pharmaceutical and food/beverage testing: Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans and Aspergillus brasiliensis (Table 2).

Samples of 10 mL sterile water were added to each filtration funnel, followed by 100 µL inoculum containing 10–100 CFU/100 µL. Sterile water was added until the total sample volume reached 100 mL. Membrane filtrations were conducted in triplicate and membranes were transferred to either TSA, R2A or SDA agar plates for incubation. Typical CFUs were counted when clearly visible and easy to identify. We then compared these results to the control spread plate counts. Mean CFU was calculated for each filtration unit and spread plate controls to determine recovery.
Results
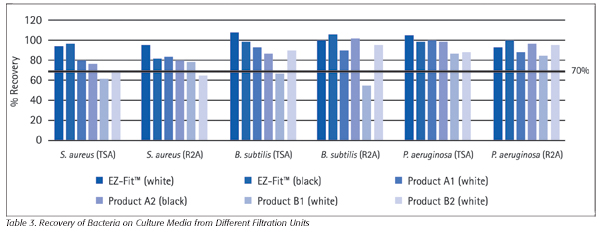 To determine effective microbiological recovery, we compared the means calculated for each filtration unit to each other, to the controls and to the regulation-mandated level of at least 70 percent of the control.[6] Across tests with all five organisms, EZ-Fit™ filtration units and two other devices exceeded these standards. A closer examination shows that results varied depending on type of microorganism (Table 3) and type of media culture (Table 4).
To determine effective microbiological recovery, we compared the means calculated for each filtration unit to each other, to the controls and to the regulation-mandated level of at least 70 percent of the control.[6] Across tests with all five organisms, EZ-Fit™ filtration units and two other devices exceeded these standards. A closer examination shows that results varied depending on type of microorganism (Table 3) and type of media culture (Table 4).

We determined that a number of variables can affect the microbiological recovery of membrane filtration devices and may have impacted these results. Each of the six products tested have cellulose-based membranes, but the ratio of cellulose acetate to cellulose nitrate differs among devices (Table 1). Across membranes with the same composition, recovery rates may be affected by sterilization processes, which should be designed and controlled to maintain optimal microbial recovery. Finally, raw material sources and culture media can also influence results.
The study also examined the general handling of each membrane filtration unit. This qualitative evaluation found that the design of the EZ-Fit™ filtration units minimized the risk of contamination and leaks by using lids that remain intact during transport and units that fit securely onto filter heads. Legible, 360° graduation and filtration funnel design also made it easy to observe the sample during the filling and filtration process using the units. Overall, these design differences had an impact on ease of handling and EZ-Fit™ filtration units showed advantages over other devices examined in the study.
Conclusion
This study demonstrates that different filtration devices vary in recovery times as a result of membrane composition, sterilization procedures and raw material sources. We also found that the design elements employed in devices can affect ease of use and handling during bioburden testing.
With changes in regulatory landscape, standards for the pharmaceutical industry are becoming more relevant for the food and beverage industry. Like pharmaceutical companies, food and beverage manufacturers face increasing demands for reliable bioburden testing and a wealth of options of testing devices. Manufacturers should carefully select the equipment they use, taking into consideration a number of variables, to ensure they are using the most efficient and effective bioburden testing devices for their needs.
Tommaso Ronconi is a global product manager of in-process testing, biomonitoring at Global Millipore SAS, Molsheim, France. Stephen M. Kuchenberg is a marketing manager, food and beverage, EMD Millipore in Massachusetts.
1. http://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm237934.htm.
3. http://www.cdc.gov/foodsafety/clostridium-perfingens.html.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →
4. http://www.rapidmicrobiology.com/test-method/theory-and-practice-of-microbiological-water-testing/.
5. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1227.html.
6. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1227.html.






