Current Trends in Mycotoxin Analysis
Fungal toxins (mycotoxins) comprise one of the major sources of food poisoning in humans, agricultural animals and wildlife. The impacts of these naturally occurring toxins range from acute disease and deaths to chronic disease with production of tumors and significant immunosuppression underlying the occurrence of other infectious bacterial and fungal diseases. Mycotoxins are formed frequently in field crops during the growing season and are unavoidable as a result of our inability to control the environmental and crop susceptibility factors that allow for their production by toxigenic, plant pathogenic fungi.
The risks associated with these mycotoxins occurring in field crops, foods, feeds and animal products impact both food safety and economic aspects of the marketing of grain for food and feed. Thus, food and feed manufacturers have a great concern for the occurrence of mycotoxins in the commodities used in their products and resort to testing for mycotoxins to provide a healthful and nontoxic product. However, among these manufacturers there is need to consider the amount of testing, the mycotoxins of concern and whether to test in-house or rely on testing of commodities and products by a commercial laboratory. For this reason, we have developed this report as an aid to understanding the analysis of mycotoxins and to assist in choosing appropriate testing measures.
The analysis of mycotoxins is the final part of overall mycotoxin testing. Sampling, subsampling and sample preparation are the first steps in the testing scheme. These first steps are where the greatest variability occurs in the overall testing scheme.[1] In this discussion, aimed at describing the function and use of the specific tests, we will assume that an adequate sample has been collected and it has been subsampled and prepared for analysis by a suitable extraction methodology for the respective mycotoxin(s)analysis. Also, we will address those methods that are most applicable in a well-developed analytical laboratory using reference methodology and discuss methodology that is generally applied to test kits that are used by feed and food manufacturers and other personnel who desire a rather rapid testing procedure. Finally, we will briefly mention some new technology that is being evaluated for analysis of mycotoxins.
ANALYTICAL METHODS MOST APPLICABLE TO AGRI-FOOD LABORATORIES
The most common analytical procedures used for mycotoxin analysis in labs include thin-layer chromatography (TLC); high-performance liquid chromatography (HPLC); gas chromatography (GC); and mass spectrometry (MS).
Thin-Layer Chromatography. TLC is the separation of compounds in a mixture that is spotted near one end of a plate (commonly glass is used as the plate) that is coated with a thin layer of an adsorbent matrix such as silica gel. Separation occurs when the end of the plate nearest the spotted mixture is placed in a solvent system in the bottom of a closed vessel (glass tank or jar) and the solvent is allowed to migrate through the adsorbent matrix and moves toward the top of the plate. As this occurs the mixture of compounds separate based on their interactions between the solvent system (mobile phase) and the matrix (stationary phase). Because these properties differ for the various compounds in a mixture, they will migrate on the plate at different rates from their starting point. If the analyte (mycotoxin in this case) of interest is present in the mixture it will be identified by comparison with standard spots of this mycotoxin that are necessarily spotted on the plate in a similar manner as the mixture.
TLC often is used as a screening technique for mycotoxins, because although it is a very powerful tool for the determination of the presence of one or more mycotoxins present in a sample, it does not allow for critical quantitation that may be required. Often, unknown mycotoxins have been found on a TLC plate that may not have been very evident using other more quantitative methods. Confirmation of the identity of a specific mycotoxin may be carried out directly on a TLC plate.
Densitometric quantitation can be conducted on a TLC plate and is more accurate when the compounds are colored or fluorescent. Thus, the analyst does not have to spray or dip the plate in a solution for visualization of the spots. TLC can often be used with little or no cleanup prior to the analysis. In some cases, two-dimensional TLC can be utilized to find mycotoxins in extremely dirty samples.
High-Performance Liquid Chromatography. HPLC is similar to TLC in principle, but is a much more sophisticated system. Basically, a small portion of a sample to be analyzed is injected into a stream of solvent being pumped through a column of an adsorptive matrix. When the sample reaches the column from the injection port it is, of course, still a mixture of compounds. As the sample moves through the column the compounds are separated by the same basic principles as described for TLC. The sample components elute off of the column as separate (it is hoped) entities, and the flow of solvent with the respective compounds passes through some sort of detector to measure the response of the specific compound. The measurements are determined based on detector response (peak height or area under the peak), which are compared to selected concentrations of standard that were injected into the instrument as part of the analysis sequence. The time for the peak appearance under the conditions of operation of the HPLC is of importance in identifying the compound of interest.
HPLC currently is the most frequently and widely used method for the analysis for mycotoxins. Methods have been developed for most of the major mycotoxins and have reasonably low levels of detection. Thus, HPLC usually is a good quantitative tool. The time for analysis of most mycotoxins is less than 15 minutes, but the methods require substantial cleanup of the extracts before injection into the system. The equipment is considerably more expensive than that required for TLC.
Gas Chromatography. GC uses sophisticated equipment in which compounds (often derivatized) are separated by a gas flowing through a heated glass column coated with a stationary nonvolatile liquid. Similar to HPLC, samples injected into the system are separated into the specific components on the column, and the separated analytes coming off of the column are detected by some chemical or physical detection system.
GC is used in more highly technical laboratories for some of the mycotoxins and is used for the analysis of the Type A trichothecenes, which do not render themselves readily amenable to HPLC analysis. This is a highly sensitive technique and the time of analysis varies with the compound of interest, but is usually less than 15 minutes. Again, the equipment is expensive and cleanup of the extract is necessary.
Mass Spectrometry. Both HPLC and GC utilize multiple, simple two-dimension detection systems that generate a chromatogram generally consisting of the concentration of an analyte as the X-axis and total detector response as the Y-axis. MS is a detection technique that can be coupled to HPLC and GC in a benchtop instrument system, and has gained significant popularity over the past decade since three dimensions of data can be acquired for an analysis. In addition to the typical chromatogram, a third dimension of information is obtained from the analytes entering the detector in the form of the mass spectrum. A mass spectrum is the “fingerprint” of a compound based on the mass to charge (m/z) ratio of the molecular ion and molecular fragments created through the ionization process. The mass spectrum is very useful in unknown identification or confirmation of the identity of peaks in a chromatogram. Mass spectrometers can also be set up to be very compound-specific detectors using selected ion monitoring in which only specific mass ions are monitored that are characteristic of the compound of interest under the conditions of analysis.
There are several types of mass spectrometers, but this article will only address those commonly used for mycotoxin analysis. The mass spectrometers used with HPLC are significantly different than those used for GC. In general, a mass spectrometer consists of an ionization source, a mass separator and an ion detection system. Ionization sources is the point at which the compounds eluting from the chromatographic column are ionized and commonly some fragmentation of the parent molecules occur. After ionization, the charged molecules and fragments are filtered by a mass separator, which allows separation of the ions based on their m/z ratio, which are then detected.
For liquid chromatography/mass spectrometry (LC-MS), the ionization sources typically are atmospheric pressure chemical ionization (APCI) or electrospray ionization (ESI), both of which are referred to as “soft” ionization techniques. The molecular ion and some molecular fragments from the parent compound are then separated by a mass separator. LC-MS is a very versatile tool in that a very wide number of compounds are suitable for detection, but a common limitation is that different compounds require different ionization conditions making general screening of samples very difficult.
For GC-MS, the historic ionization source for both identification and quantitation is electron impact (EI), which is called a “hard” ionization technique. As the compound elutes from the CC column, the molecules are bombarded with high-energy electrons. The charge induced on the molecules commonly causes them to fragment significantly. This fragmentation leads to the characteristic EI mass spectra which is very useful in the identification of unknowns either by mass spectral interpretation, or by comparison of the fragmentation pattern with commercial spectral libraries. Methods that utilize another ionization technique, negative chemical ionization (NCI), are becoming more common in the literature due to remarkable sensitivity and low background noise. After ionization, the fragments are separated based on their m/z ratio and detected. GC-MS is a very versatile tool for screening samples for unknowns, as well as for the quantitation of compounds suitable for CC analysis.
METHODS USED PRIMARILY FOR TEST KITS
The methods used in mycotoxin test kits are primarily designed to achieve a rapid analysis of mycotoxins occurring in commodities and some products derived from them. The primary types of procedures used are enzyme-linked immunosorbent assay (ELISA), affinity column chromatography and fluorometry.
Enzyme-linked immunosorbent assay. This is one of the most popular immunologically based methods used in test kits for the analysis of mycotoxins in foods and feeds. Most methods employed for mycotoxins use monoclonal antibodies specific for the mycotoxin in the assay. Often, ELISA methods will give quantitative answers greater than that by HPLC, because the antibodies may cross-react with other compounds of similar structure in the sample. Once a test has been developed, the method is quite easy to use and the equipment necessary is relatively inexpensive.
The technique for conducting an ELISA may use one of several possible approaches, but a commonly used approach is a competitive assay in which a known amount of labeled (enzyme) toxin competes with any possible toxin in the sample for the specific antibodies attached to the reaction vessel (usually a microtiter well or a test tube). Any unbound toxin is washed from the vessel. The quantitation is dependent upon the amount of enzyme-labeled toxin remaining in the vessel to react with the substrate for the enzyme. This reaction results in a colored product that can be measured optically. Therefore, a highly colored reaction would indicate the absence of the specific mycotoxin in the sample.
Affinity Column Chromatography. Affinity column chromatography has been used for quite a few years in the analysis of mycotoxins. Essentially, the technique is used to clean up extracts prior to an analytical procedure used to measure the mycotoxin. In these columns, monoclonal (usually) antibodies specific for a given toxin are attached to the column matrix. When an extract is placed on the column and the column is washed, only the specific toxin remains on the column as it has bound to the antibody. The attached toxin is then eluted from the column by changing the solvent system to 90% methanol in water; thus, the eluate contains rather pure toxin, which can be analyzed using any technique such as fluorometry or HPLC.
It is possible to apply more than one antibody to the column for use in simultaneous isolation of more than one mycotoxin. This has been applied to fumonisin B1 and its hydrolysis product prior to HPLC analysis. This potential exists for other mycotoxins, as well.
Fluorometry. The basis of fluorometry is the quantification of compounds by measuring their fluorescence using a fluorometer. In some cases, the compounds may be innately fluorescent, while in others, the compounds are rendered fluorescent through some chemical derivatization. The tests for compounds using fluorometry includes the extraction of the compound from the specific matrix, followed by a cleanup process using either affinity columns or solid phase cleanup columns, and then derivatization (if necessary) and measurement of the fluorescence. The equipment for fluorometry is rather inexpensive, since the fluorometer is a one-time expense and the test kits usually contain most of the necessary materials to run the test.
CURRENT TECHNOLOGY IN DEVELOPMENT
One of the newer technologies applied to the analysis of mycotoxins is capillary electrophoresis (CE). Using this technique, a mixture of compounds having dissimilar charges can be separated in a capillary column using electrophoresis. Because of the use of capillary columns, small sample volumes can be used for analysis and thus eliminates the use of large quantities of hazardous solvents. This method is not as easy to perform as HPLC, requires considerable clean up, requires longer derivatization time, at least for some mycotoxins, and the equipment is quite expensive. However, it is rapid, the capillary columns are easily regenerated with rinsing and only small amounts of reagents are required for the test.
Cell-based assays are often employed in the analysis for mycotoxins because they reflect both toxin interaction with the receptor on the cells and the subsequent intracellular responses of the cell that result from the toxin/receptor interaction. Toxin class specificity can be achieved, at least to some extent, by choosing a particular cell line known to express a given response to a particular toxin or class of toxins. Endpoints in these assays include cytotoxicity or quantifiable changes in cell responses.
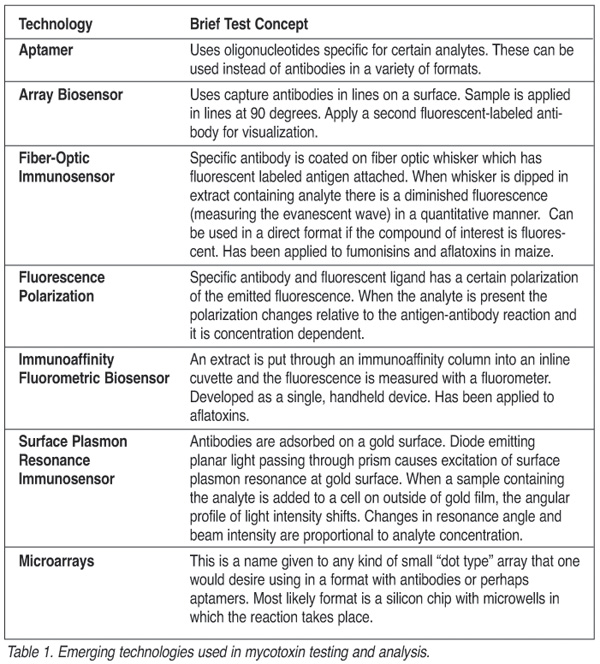
Currently, a developmental strategy for many natural products and pesticides is the use of biosensors. These biosensors utilize diverse technological advances such as monoclonal antibodies, optics, microchips and recombinant genetic materials put together in combinations to achieve rapid, specific and quantitative measurement of the desired analytes. Table 1 describes some of the emerging techniques in the field of mycotoxin analysis that are in research status with no test kits or only limited applications for mycotoxins available commercially.
Our ability to control and manage the contamination of mycotoxins in feeds and foods is dependent upon the development of procedures for analyzing mycotoxins in them. These methods must be valid, efficient, sensitive and accurate to make the world’s food safer.
John L. Richard, Ph.D., is Chief Executive Officer of Romer Labs, Inc., based in Union, MO, a leading mycotoxin control consulting laboratory. Romer Labs has nearly 20 years of experience in the analysis of mycotoxins using reference methods employing TLC, HPLC, and GC/MS analyses. Previously, Richard headed mycotoxin research at the National Center for Agricultural Utilization Research in Peoria, IL, where he was responsible for a $2 million budget for USDA mycotoxin control programs for agricultural commodities.
Keith Fleetwood, is an analytical chemist in the Technical Services Department of Romer Labs, Inc. He has extensive experience with a wide variety of analyses for mycotoxins, including SPE columns, immunoaffinity columns and solvent separation methods, as well as HPLC, TLC and titration methods.
REFERENCE
1. Romer Labs, Inc. Guide to Mycotoxins, Vols. 1-3. Union, MO. January 2000, July 2000, October 2000.
Looking for quick answers on food safety topics?
Try Ask FSM, our new smart AI search tool.
Ask FSM →







