The U.S. Food and Drug Administration has announced a new, transformative vision for the agency’s Human Foods Program, as well as for the Office of Regulatory Affairs (ORA) to better support FDA as a whole. The plan includes creating a unified Human Foods Program under a single leader who reports directly to the Commissioner of FDA, removing redundancies and enabling the agency to oversee human food in a more effective and efficient way.
The agency’s decision comes after reviewing the findings and recommendations from an external evaluation conducted by the Reagan-Udall Foundation, as well as a separate internal review of the agency’s infant formula supply chain response completed last year. The findings and recommendations from these reviews identified issues surrounding culture, structure, resources, and authorities at FDA. They also noted several areas of need, including modernizing data systems, providing more resources and authorities, improving emergency response systems, and building a more robust regulatory program.
In the agency’s briefing on the new vision, FDA Commissioner Robert M. Califf, M.D. explained that the need for a new approach to FDA’s human foods activities was apparent upon his return to the agency, which was shortly before the infant formula supply crisis arose. The transformation of FDA was put on hold while the crisis was handled. Additionally, FDA Principal Deputy Commissioner Janet Woodcock, M.D. explained that the infant formula supply crisis and the breakdown that occurred in the agency’s response was a “systems problem, not an individual problem,” and that individuals will not be asked to leave their position or be reassigned as a way of demanding individual accountability for the crisis.
Also in the briefing, concerns about the new FDA structure adequately mitigating future shortages of critical foods were raised. Dr. Califf explained that the new structure is intended to forge a more preemptive approach to mitigating possible issues, realizing the vision of the FDA Food Safety Modernization Act. However, he explained that supply chain issues still exist and are something that the entire country will need to address, stating, “although FDA is raising the issue [of supply chain challenges], it is not within FDA’s purview.” Dr. Woodcock added that, while FDA will do everything in its power to prevent a shortage of a critical food in the future, it could still happen. She explained that, unfortunately, shortages are always a risk for products with a limited number of suppliers, and provided the example of similar situations occurring within the U.S. drug supply.
The proposed structures for both the Human Foods Program and ORA will have clear priorities that are focused on protecting and promoting a safe, nutritious U.S. food supply, with the ability to more quickly adapt to an ever-changing and evolving environment. The new approach is intended to address the recommendations outlined in the aforementioned reports on FDA’s performance, and takes into consideration feedback from stakeholders, as well from employees working in the Human Foods Program who had an opportunity to share input through numerous interactive and listening sessions over the past month.
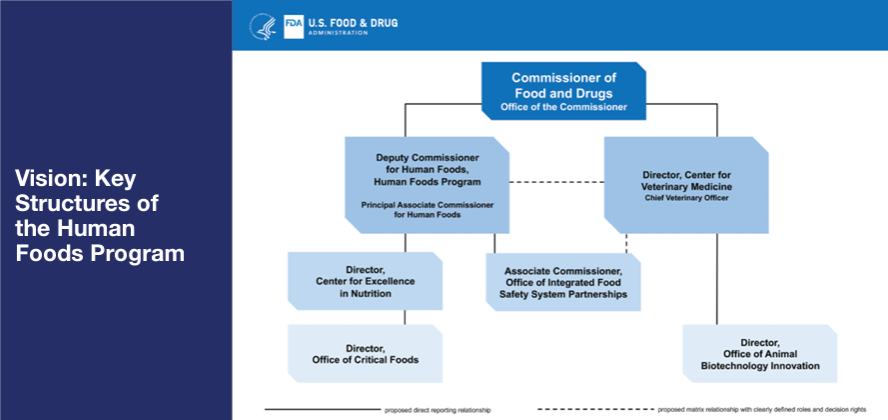
Under the new plan, the functions of the Center for Food Safety and Applied Nutrition (CFSAN), the Office of Food Policy and Response (OFPR), and certain functions of ORA will be unified into a newly envisioned organization called the Human Foods Program (Figure 1). FDA will conduct a competitive national search for a Deputy Commissioner for Human Foods who will oversee the program. The person in this position will report directly to Dr. Califf and will be charged with leading a unified Human Foods Program that keeps regulated foods safe and nutritious, while ensuring the agency remains on the cutting edge of the latest advancements in science, technology, and nutrition. The Deputy Commissioner will have decision-making authority over policy, strategy, and regulatory program activities within the Human Foods Program, as well as resource allocation and risk-prioritization. Other key elements of the proposed new Human Foods Program organization include:
- The creation of a Center for Excellence in Nutrition that prioritizes FDA’s ongoing efforts to help American consumers make more informed food choices, including by working with industry to offer healthier, more nutritious food products. FDA proposes to establish an Office of Critical Foods, as directed by the 2023 Consolidated Appropriations Act, within this center.
- The establishment of an Office of Integrated Food Safety System Partnerships that will focus on elevating, coordinating, and integrating FDA’s food safety and response activities with state and local regulatory partners to more effectively meet the vision of an Integrated Food Safety System as envisioned in FSMA. This newly proposed structure will ensure greater collaboration and support of state-level inspectional activities.
- To help support the agency’s scientifically grounded decision-making activities, a Human Foods Advisory Committee will be established. The Human Foods Advisory Committee will consist of external experts to advise FDA on challenging and emerging issues in food safety, nutrition, and innovative food technologies.
- There will be an emphasis on strengthening FDA’s enterprise information technology and analytical capabilities to fulfill the promise described in the New Era of Smarter Food Safety and support the improvement in workflow that will accompany these changes. This area of focus will support the work of the Human Foods Program by enabling more facile communication, more efficient operations, and enhanced empirical risk algorithms to guide the priorities of the program and the work in the field.
Figure 1. FDA’s Vision for Key Structures of the Human Foods Program.
Image credit: The U.S. Food and Drug Administration
As part of the proposed new vision, ORA’s operating structure will be transformed into an enterprise-wide organization that supports the Human Foods Program and all other FDA regulatory by focusing on its critical activities. This realignment will allow ORA to be singularly focused on excellence in its core mission—inspections, laboratory testing, import, and investigative operations—by improving risk prioritization and public health impact of FDA’s field activities, modernizing FDA’s field activities, and creating operational efficiencies. This will optimize ORA’s operations in line with FDA’s public health and prevention-oriented goals. Certain functions of ORA will be aligned with other parts of FDA to create an overall stronger agency.
In the briefing, Dr. Califf and Dr. Woodcock elaborated that boots-on-the-ground inspectors will remain within ORA, but the priorities and budget for human foods-related activities will be the direct responsibility of the new Deputy Commissioner of Human Foods. Although the details are still being set, FDA expects a written agreement will be laid out that describes who gets to make certain decisions about budget, strategy, and difficult policy issues, reflective of a less hierarchical organizational structure. “You don’t need to have one person at the top [of a pyramid of subordinates] to make this work,” Dr. Woodcock stated in response to concerns about human foods activities being prioritized at ORA.
While the FDA’s Center for Veterinary Medicine (CVM) will continue to operate as a standalone center, the relevant food safety activities will be closely coordinated between the CVM Center Director and Deputy Commissioner for Human Foods. The CVM Director role will also be expanded to strengthen the center’s One Health role and connection to the Human Foods Program. This proposed structure will allow CVM to support the Human Foods Program where its activities are relevant to human food safety. Additionally, an office of Animal Biotechnology Innovation within CVM will be created to advance the FDA’s ongoing smart regulation of animal biotechnology.
FDA has recently formed an Implementation and Change Management Group that will be charged with developing a detailed plan to ensure the successful execution of the new vision.
As a next step, FDA will need to develop the vision into a concrete reorganizational proposal. While details of this proposal continue to be developed, CFSAN, ORA, and OFPR will continue to operate under their current structures, Dr. Califf’s direct oversight. Additional public updates on FDA’s progress, organizational design, and timeline are expected by the end of February 2023.




