In Part I of this article,[1] it was revealed that spices are the subject of increasing recalls for food pathogens. This article focuses on what the industry can do. My perspective is that nothing short of a world-class food safety system will be adequate for U.S. spice processors to prevent and survive after such recalls.
Supplier Controls
I previously showed that spices may be produced under unsanitary conditions in Third World countries. Very often, lot identity is lost as shipments move from country to country. International spice trade organizations and the U.S. food industry should be involved in enhancing lot tracking efforts and developing and insisting upon appropriate supplier controls, including adherence to Good Agricultural Practices and compliance with the Food Safety Modernization Act.
In many instances, microbiological control includes a step, often at the port of entry into the U.S., to reduce the microbial population in spices, using irradiation, ethylene oxide, propylene oxide and steam treatments. However, validation of these techniques is essential, recognizing that a treatment that effectively reduces 5–6 log CFU on one spice may have a different effect on another, due to differences in the matrix, packing density, water activity (aw), etc. Niemira[2] reviewed the effects of irradiation, microwave and alternative-energy-based treatments for low-aw foods, and Grasso et al.[3] reviewed the impact heat and steam treatments have on such foods. However, the microbial load on spices may be quite high, with 3% of dried herbs and spices having Bacillus cereus counts greater than or equal to 100,000 per gram4 and 1.5% and 1.1% Salmonella contamination of dried spices and herbs sampled at retail and production, respectively, out of 2,833 retail samples and 132 production batches tested.
Lot Selection and Testing for Pathogens
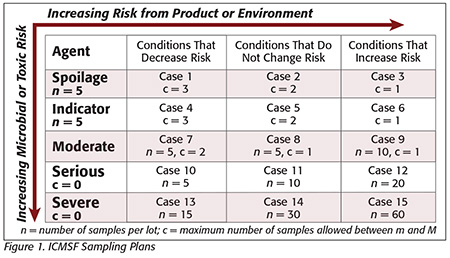 Often, spices are purchased based on lot selection, for example, testing microbial counts or pathogen presence or absence. The distribution of microbial populations is rarely uniform and often sporadic, especially in a dry powdered product.[5,6] Even when contamination happens, the microbial populations are normally distributed when converted into logarithms, as opposed to a standard distribution. This nonuniformity should be recognized by purchasers of spices, who should insist that if “lot selection” is used, an appropriate sampling and testing plan is in place. A test from a single sample taken from a lot is not adequate. Such a plan would take into consideration the risk associated with the organism tested and whether risks increase, decrease or remain the same during storage, handling and distribution. Such plans are provided by the International Commission for the Microbiological Specifications for Foods (ICMSF, Figure 1),[7] the U.S. Food and Drug Administration (FDA) Bacteriological Analytical Manual (BAM)[8] and certain European plans, as summarized by Sagoo et al.,[4] shown in Figure 2. A discussion of these plans was also previously published.[9]
Often, spices are purchased based on lot selection, for example, testing microbial counts or pathogen presence or absence. The distribution of microbial populations is rarely uniform and often sporadic, especially in a dry powdered product.[5,6] Even when contamination happens, the microbial populations are normally distributed when converted into logarithms, as opposed to a standard distribution. This nonuniformity should be recognized by purchasers of spices, who should insist that if “lot selection” is used, an appropriate sampling and testing plan is in place. A test from a single sample taken from a lot is not adequate. Such a plan would take into consideration the risk associated with the organism tested and whether risks increase, decrease or remain the same during storage, handling and distribution. Such plans are provided by the International Commission for the Microbiological Specifications for Foods (ICMSF, Figure 1),[7] the U.S. Food and Drug Administration (FDA) Bacteriological Analytical Manual (BAM)[8] and certain European plans, as summarized by Sagoo et al.,[4] shown in Figure 2. A discussion of these plans was also previously published.[9]
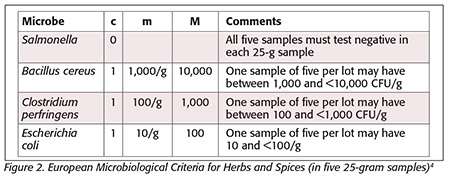 European criteria[4] indicate that five samples (n) per lot be tested for Salmonella, B. cereus, Clostridium perfringens and generic Escherichia coli. In this scheme, c (the number of samples allowed above m and below M) is 0 for Salmonella, with all five samples tested at the 25-g level. Since c = 0 for Salmonella, no sample may test positive and still be acceptable.
European criteria[4] indicate that five samples (n) per lot be tested for Salmonella, B. cereus, Clostridium perfringens and generic Escherichia coli. In this scheme, c (the number of samples allowed above m and below M) is 0 for Salmonella, with all five samples tested at the 25-g level. Since c = 0 for Salmonella, no sample may test positive and still be acceptable.
The ICMSF scheme (Figure 1) and the FDA BAM approach would suggest that n should be 15, 30 or 60, depending on the end use of the product, for Salmonella, unlike the European approach, which had five samples tested per lot. It should be noted that if thirty 25-g samples were selected, they could be composited into two 375-g test samples per FDA’s BAM.[8] Lots would need to be rejected or treated with a validated approach to destroy Salmonella if any samples from the lot tested positive.
Aerobic Plate Count (APC) Suggestions
Sometimes, a spice purchaser will establish a “low bac” specification of their own choosing. This amounts to a value of M in the ICMSF and European schemes. However, it should not be assumed that because a value of M is assigned that it is the actual maximum level in the lot. In other words, setting a value of M is not a guarantee that such levels are not present.
For example, assume that the purchaser of spices wishes to have a lot with fewer than 100,000 CFU/g for APC and they receive results of 50,000, 10,000, 70,000, 80,000 and 5,000 from five subsamples. All these values are actually less than 100,000, but is the maximum level in the lot really under 100,000 CFU/g? Transforming the data into log values to reduce the variation and account for potential log10 normal contamination results in values of 4.70, 4.00, 4.84, 4.90 and 3.70 log CFU, respectively. The average log10 CFU is thus 4.41 log CFU/g with a standard deviation of 0.49. A 95% confidence interval is approximated by ±2× the standard deviation on either side of the mean, or 0.98 in this example. Adding 0.98 to 4.41 results in 5.39 log, which exceeds our specification of 100,000 CFU/g (5 log), and the lot is very likely to have a maximum APC greater than 100,000 CFU/g, that is, closer to 260,000 CFU/g.
The best approach is to work this backward. Thus, one could add 2 or 3 standard deviations to the average of the log10 CFU/g values to set M in the case of testing for nonpathogens.
Note that if the standard deviation of the log values collected over time is 0.50 and one does not wish to receive a lot with a maximum value of more than 100,000 CFU/g, then the specification for M could be set at 5.00–1.00 (2× the standard deviation of 0.5) or 4.00 log (10,000 CFU/g). In this example, setting M equal to 10,000 CFU/g ensures that 95% of the time, the lots received should be below 100,000 CFU/g.
Appropriate Sanitization and Regulatory Philosophy
 FDA regards any low-dose pathogen (Salmonella, Shiga toxin-producing E. coli, Listeria monocytogenes)-contaminated ready-to-eat food processed on the same line or portions of a line between verified cleaning and sanitization breaks to be adulterated.[10] Hence, any manufacturing lots included in this “regulatory lot” in the marketplace would be subject to recall. This highlights the importance of cleaning and sanitization to create a “clean break” and of segregating processing lines (Figure 3[11]). Thus, food produced before or after clean breaks may not be subject to recall if appropriate assurances can be provided that it is also not adulterated. But before we move on, some definitions are in order.
FDA regards any low-dose pathogen (Salmonella, Shiga toxin-producing E. coli, Listeria monocytogenes)-contaminated ready-to-eat food processed on the same line or portions of a line between verified cleaning and sanitization breaks to be adulterated.[10] Hence, any manufacturing lots included in this “regulatory lot” in the marketplace would be subject to recall. This highlights the importance of cleaning and sanitization to create a “clean break” and of segregating processing lines (Figure 3[11]). Thus, food produced before or after clean breaks may not be subject to recall if appropriate assurances can be provided that it is also not adulterated. But before we move on, some definitions are in order.
Cleaning is the process that removes soil. Some soils such as protein and fat can protect microbes from the effect of aqueous sanitizer, much like an umbrella protects its user from the rain. One cannot effectively sanitize a surface that is not effectively cleaned. Sanitization is the treatment that destroys vegetative (non-spore-forming) microbes of public health significance and substantially reduces the numbers of other undesirable microorganisms.
The removal of soil should be assessed visually and any unclean surfaces recleaned immediately. In addition, those surfaces in which wet cleaning is indicated can also be tested for adenosine triphosphate (ATP), protein-based assays or other suitable tests. ATP and protein-based swab approaches are likely to be unsuitable for dry cleaning validation. It should be recognized that ATP, while appropriate for assessment of wet cleaning efficacy on the sites sampled, is not appropriate for monitoring the efficacy of sanitization.[12]
Dry vs. wet cleaning
Those industries that manufacture high-moisture products unlike spices have designed equipment that can accommodate water.[10] Poorly designed equipment that traps wet product residues results in contamination of products produced on such lines, with spoilage organisms leading to loss of consumer confidence. Low-aw foods like spices are regarded as low-risk due to lack of microbial growth and spoilage, but this perception has changed in the past decade with numerous instances of low-aw food pathogen-associated recalls or outbreaks.[10] The low-risk perception associated with low-aw foods resulted in equipment that was rarely wet-cleaned and sanitized, and was often not designed to be wet-cleaned and sanitized. However, increased perception of risk as well as the need for allergen control have resulted in more frequent wet cleaning of such equipment, which results in more, not less, microbiological risk.[10] Consequently, dry approaches to cleaning and sanitizing are needed.
Dry cleaning approaches
Dry cleaning approaches can include sweeping, wiping, vacuuming and dry-ice blasting. Blowing residues with compressed air is discouraged due to aerosolization of contaminants.[13]
Dry sanitization approaches
Wet cleaning and sanitization increases microbiological risk in dry processing areas and on equipment used to process low-aw foods. What is appropriate for one piece of equipment may not be appropriate or practical for another. The following is a list of “dry” sanitization approaches:
• Damp-mopping floors with sanitizer and restricting access to them during drying is a simple approach and may be considered a “dry” sanitization.
• CO2-entrained alcohol can be used for sanitizing areas of the facility. Alcohol provides an immediate lethality to populations of vegetative microorganisms but then evaporates quickly, leaving surfaces dry.
• Wiping selected pieces of equipment with alcohol wipes can be done. One must exercise appropriate caution if in areas with risk of flammability or in confined spaces.
• If an alcohol-based quaternary ammonium sanitizer is used, one gets the immediate alcohol-associated lethality, leaving behind a quaternary ammonium sanitizer residue, which if wetted due to processing activities will become germicidal.
• Steaming pieces of equipment to achieve 160 °F wet heat for at least 45 minutes14 is also useful to destroy biofilms of L. monocytogenes. The heat from steaming may drive off residual moisture from equipment, as has been observed in steam chambers, which I have assisted in validating.
• Gaseous disinfection approaches such as chlorine dioxide or ozone gases have been used. Some third parties provide these services. A similar concept is vapor-phase hydrogen peroxide. However, all such approaches need to be performed with appropriate safety precautions in place and should be validated for their efficacy with appropriate biological indicators.
Environmental Sampling and Corrective Action Programs
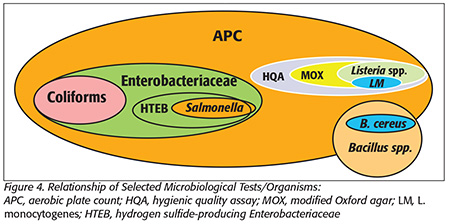 Salmonella is the principal organism of concern for spices and most low-aw foods, given its low infectious dose and ability to survive in low-aw environments for long periods.[15–17] Consequently, an environmental monitoring program should include Salmonella, which will facilitate determination of the efficacy of hygienic activities conducted in the plant. Companies also sample their production and near-production environments for Enterobacteriaceae (EB) counts and/or the presence/absence of hydrogen sulfide-producing EB (HTEB). The relationship between these assays is shown in Figure 4.
Salmonella is the principal organism of concern for spices and most low-aw foods, given its low infectious dose and ability to survive in low-aw environments for long periods.[15–17] Consequently, an environmental monitoring program should include Salmonella, which will facilitate determination of the efficacy of hygienic activities conducted in the plant. Companies also sample their production and near-production environments for Enterobacteriaceae (EB) counts and/or the presence/absence of hydrogen sulfide-producing EB (HTEB). The relationship between these assays is shown in Figure 4.
One typically finds HTEB before one finds Salmonella in a plant environment. Hence, early eradication of HTEB-positive sites is a tool that may prevent such sites from developing into Salmonella growth niches. HTEB sites with high EB counts are of particular concern. A listing of laboratories that provide HTEB testing can be found online.[18]
Tracking and trending Salmonella, HTEB and/or EB sites should be done with documentation of corrective actions taken. Documenting corrective action is one means of demonstrating that the facility has a food safety culture.
Preoperational Sampling Programs
Verification of the sanitization break described earlier should be documented, including identifying who performed cleaning and sanitization, what cleaning and sanitizing chemicals were used and their concentrations. Standard Operating Procedures for all cleaning and sanitizing steps (e.g., clean in place, clean out of place of removable parts and manual cleaning of stationary equipment) as well as the plant environment (ceilings, walls, floors, etc.) should be kept up to date with appropriate documented change policies. In addition, data should be generated that provide evidence that sanitization was effective. This can take the form of preoperational swabs for appropriate hygienic indicator organisms (e.g., EB and APC). In some instances, particularly after a regulatory event such as a recall, it may be appropriate to swab surfaces for the target organism (e.g., Listeria spp. or Salmonella) for a time, provided that product produced on those surfaces is held pending acceptable results. However, it should be recognized that a pathogen recovered from a preoperational surface implicates production lots on either side of the clean break.
Segregation of Lines in Time and Space
Remember, FDA regards any low-dose pathogen-contaminated ready-to-eat food processed on the same line or portions of a line between verified cleaning and sanitization breaks to be adulterated. Given that products produced on common lines or parts of lines can be implicated in recall events, segregating lines associated with different products is encouraged.
Hygienic Equipment Design and Repair Practices
Increasing pressures on the low-aw food processing industry to perform wet cleaning should be resisted unless appropriate equipment and facilities are designed to accommodate water as described earlier. The spice industry should insist that its suppliers of equipment follow appropriate hygienic design criteria, such as that provided by the Grocery Manufacturers Association (GMA).[19] GMA facility design criteria for low-aw food processing can be found online.[20]
Preventive Maintenance Programs
In my experience, most microbial contamination events in the low-aw food industry occur because of either poor sanitary design or unhygienic preventive maintenance programs. The spice industry would be well served to periodically review its own programs for performing preventive maintenance. The industry is encouraged to engage its food safety and quality professionals in this effort.
Ongoing Training
Bacteria are about 1 million times smaller than humans in every dimension and invisible. They can penetrate many areas that seem impossible to those not expecting such a profound difference in relative size. Small cracks and crevices, and even multiple layers of gaskets (e.g., on an air-operated valve stem), may permit microbes to migrate into food streams. Awareness of these potentials through appropriate training, rightly applied, can be used to prevent future recalls in the new regulatory environment in which the spice industry finds itself.
Jeffrey L. Kornacki, Ph.D., is president of Kornacki Microbiology Solutions Inc. in Madison, WI. He is on the Editorial Advisory Board of Food Safety Magazine.
References
1. www.food-safety.com/magazine-archive1/december-2016january-2017/the-coming-storm-in-the-spice-industry/.
2. Niemira, BA. 2014. “Irradiation, Microwave, and Alternative Energy-Based Treatments for Low-Water Activity Foods,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds. Gurtler, JB, MP Doyle and JL Kornacki (New York: Springer), 389–401.
3. Grasso, EM et al. 2014. “Steam and Heat Treatments,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds. Gurtler, JB, MP Doyle and JL Kornacki (New York: Springer), 403–424.
4. Sagoo, SK et al. 2009. “Assessment of the Microbiological Safety of Dried Spices and Herbs from Production and Retail Premises from the United Kingdom.” Food Microbiol 26:39–43.
5. Kornacki, JL. 2010. “How Many Samples Do I Take?” in Principles of Microbiological Troubleshooting in the Industrial Food Processing Environment, ed. Kornacki, JL (New York: Springer), 137–146.
6. Courdier, JL. 2014. “Methodological and Sampling Challenges in Testing Spices and Low-Water Activity Food for the Presence of Foodborne Pathogens,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds. Gurtler, JB, MP Doyle and JL Kornacki (New York: Springer), 367–386.
7. ICMSF. 2002. Microorganisms in Foods 7: Microbiological Testing in Food Safety Management (New York: Kluwer Academic Plenum Publishers).
8. www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm063335.htm.
9. Kornacki, JL. 2011. “Practical Sampling Plans, Indicator Microorganisms, and Interpretation of Test Results from Microbiological Troubleshooting,” in Rapid Detection and Quantification of Foodborne Pathogens, ed. Hoorfar, J (Washington, DC: ASM Press), 373–379.
10. Kornacki, JL. 2012. “Hygiene Control in the Dry Food Products Industry: The Roles of Cleaning Methods and Hygienic Indicators,” in Case Studies in Food Safety and Authenticity: Lessons from Real-Life Situations, ed. Hoorfar, J (Cambridge, UK: Woodhead Publishing), 254–266.
11. Stanfield, P. 2003. “Cleaning and Sanitizing a Food Plant,” in Food Plant Sanitation, eds. Hui, YH, BL Bruinsma, JR Gorham, WK Nip, PS Tong and P Ventresca (New York: Marcel Dekker), 101–114.
12. Kornacki, JL. 2010. “How Do I Sample the Environment and Equipment?” in Principles of Microbiological Troubleshooting in the Industrial Food Processing Environment, ed. Kornacki, JL (New York: Springer), 125–136.
13. www.food-safety.com/magazine-archive1/junejuly-2014/airborne-contamination-a-microbiologiste28099s-perspective/.
14. Chmielewski, RA and JF Frank. 2004. “A Predictive Model for Heat Inactivation of Listeria monocytogenes Biofilm on Stainless Steel.” J Food Prot 67(12):2712–8.
15. Gurtler, JB, MP Doyle and JL Kornacki. 2014. “The Microbiological Safety of Spices and Low-Water Activity Foods: Correcting Historic Misassumptions,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds. Gurtler, JB, MP Doyle and JL Kornacki (New York: Springer), 3–13.
16. Kornacki, JL. 2014. “Processing Plant Investigations: Practical Approaches to Determining Sources of Persistent Bacterial Strains in the Industrial Food Processing Environment,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds. Gurtler, JB, MP Doyle and JL Kornacki (New York: Springer), 67–83.
17. Bean, DC and LS Post. 2014. “Chocolate and Confectionary,” in The Microbiological Safety of Low Water Activity Foods and Spices, eds. Gurtler, JB, MP Doyle and JL Kornacki (New York: Springer), 269–293.
18. www.kornackifoodsafety.com.
19. www.gmaonline.org/downloads/technical-guidance-and-tools/GMA_Equipment_Design_Checklist_05_14_2010.xls.
20. www.commercialfoodsanitation.com/v2/wp-content/uploads/2015/03/Download-GMA-Facility-Checklist-for-Low-Moisture-Foods-EN.xls.




