The spice industry is poised on the edge of major regulatory interventions, and the result will be profound changes in its food safety systems, particularly as they relate to contamination with pathogenic microorganisms, if the industry is to survive. This opinion is drawn from the use of new surveillance tools coming onboard at the U.S. Food and Drug Administration (FDA) and the U.S. Centers for Disease Control and Prevention (CDC), increasing FDA involvement with spices, principally with potential Salmonella contamination and growing regulatory, academic and industry awareness of Salmonella risk with low water activity foods, spices and “stealth” foods.
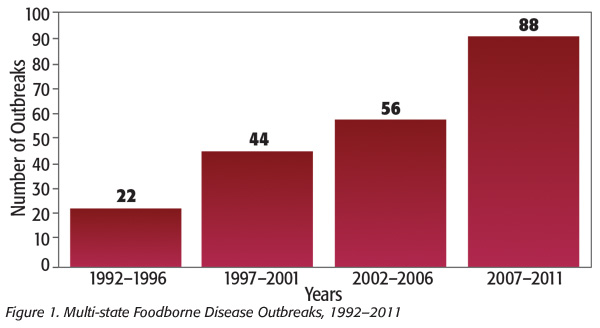 The food industry has gone through a great change with the introduction of the FoodNet/PulseNet surveillance in the late 1990s, resulting in smaller but more numerous and widely dispersed outbreaks (Figure 1).
The food industry has gone through a great change with the introduction of the FoodNet/PulseNet surveillance in the late 1990s, resulting in smaller but more numerous and widely dispersed outbreaks (Figure 1).
This upward outbreak trend is in spite of reductions or leveling off of actual foodborne bacterial illnesses (Figure 2).
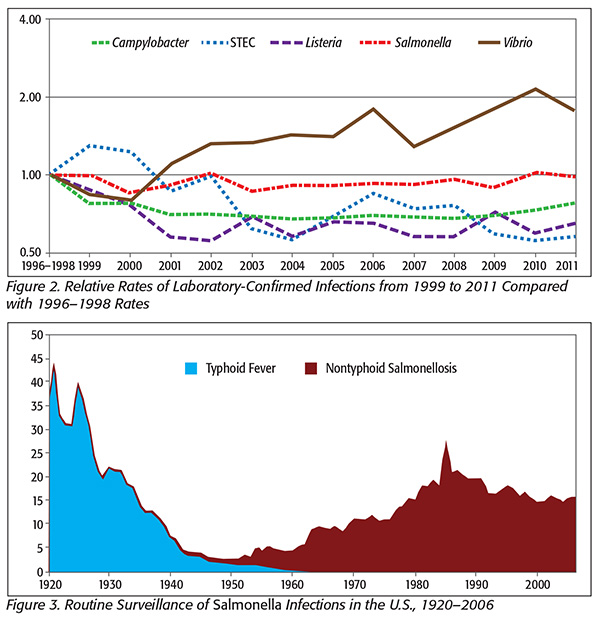 In Figure 3, note that Salmonella illnesses have remained constant in spite of the increased surveillance. Furthermore, the estimated incidence of Salmonella infection is incredibly high compared with most other bacterial infections.
In Figure 3, note that Salmonella illnesses have remained constant in spite of the increased surveillance. Furthermore, the estimated incidence of Salmonella infection is incredibly high compared with most other bacterial infections.
Salmonella infections are estimated at well over 1 million cases per year, with most believed to be foodborne.[1,2] The high estimate and comparatively low discovery of outbreaks have caused some to speculate that these illnesses come from so-called stealth foods, for example, those that people are unlikely to remember. CDC is quoted thus: “Examples include garnishes, condiments and ingredients that are part of a food item used at low levels in a wide variety of foods such as spices.”[3]
This lack of Salmonella outbreak detection is about to change. One reason is that a new surveillance approach has been launched in the U.S. in which the current technology, pulsed field gel electrophoresis used in FoodNet surveillance, is being replaced by whole-genome sequencing (WGS). The impact of this change has already been profound. WGS was recently introduced to track Listeria infections. About 3 years ago, CDC was detecting about three outbreaks of listeriosis every year. This was up from about one outbreak every 3 years prior to the introduction of FoodNet/PulseNet in the late 1990s. Last year, 10 Listeria outbreaks were found.[4] This increase in Listeria outbreak detection over the pre-FoodNet/PulseNet era is likely to further increase as the government adds strains to its database (often collected during visits to manufacturing facilities) and more resources as mandated by the Food Safety Modernization Act. Some have predicted that this may increase Listeria outbreak detection 50- to 100-fold over the pre-FoodNet/PulseNet era. At present, this translates to a 3.3-fold increase in the number of outbreaks detected per year over the average number of Listeria outbreaks detected only 3 years ago. This number will probably increase two- to fourfold in coming years. But what has that got to do with Salmonella?
 There are about 1,000-fold more Salmonella cases in the U.S. than Listeria cases. In fiscal 2017, the federal government is poised to introduce WGS surveillance to Salmonella. The government already has a library of about 35,000 draft whole-genome sequences of Salmonella in its GenomeTrakr database. This appears to be over four times the number of the sequences for Listeria in the GenomeTrakr database (Figure 4[5]).
There are about 1,000-fold more Salmonella cases in the U.S. than Listeria cases. In fiscal 2017, the federal government is poised to introduce WGS surveillance to Salmonella. The government already has a library of about 35,000 draft whole-genome sequences of Salmonella in its GenomeTrakr database. This appears to be over four times the number of the sequences for Listeria in the GenomeTrakr database (Figure 4[5]).
The result is that given increased awareness of the risk of spice contamination with Salmonella and enhanced surveillance technologies, many previously unrecognized foods are likely to be found contaminated with Salmonella. The following provides evidence of increased governmental awareness of the potential risk associated with spices.
FDA Involvement with Spices
Vij et al.[6] surveyed 21 spice recalls across 12 spice types in the United States from 1970 to 2003. A review of these data suggests a dramatic increase in U.S. recalls over this period, with 76 percent of the recalls occurring in the last 4 years of the study and the remainder occurring over the preceding 30 years. All recalls except one were due to Salmonella contamination.
FDA regards spices contaminated with Salmonella to be adulterated.[6,7] In 2013, FDA’s Center for Food Safety and Applied Nutrition published its Draft Risk Profile: Pathogens and Filth in Spices document,[7] which quotes a study by Ma[8] that indicates that this trend of increasing spice recalls is continuing, with eight recalls occurring in the 2-year period from January 2008 through December 2009. These recalls were associated with actual or potential Salmonella contamination and involved 116 different products. FDA indicated that lack of supplier control was a contributing factor. However, Ma[8] reported that insufficient/inadequate sanitation controls, environmental monitoring and training among processors were root causes for recalls associated with a white pepper outbreak.
Recalls and Illnesses Associated with Spices in Other Foods
Low numbers of Salmonella spp. were associated with paprika-powdered potato chips in Germany that resulted in about 1,000 cases of salmonellosis in 1993 from a wide variety of serotypes. Paprika imported from South America was implicated. Salmonella levels in the spice blend associated with this outbreak ranged from 0.04 to 11 most probable number/g,[7] low numbers indeed.
A large recall was issued in 2010 for ready-to-eat salami products contaminated with Salmonella Montevideo/Senftenberg from contaminated black and red pepper that resulted in 234,686 pounds of salami products being recovered from the marketplace.[9] Approximately 1.3 million pounds of a low water activity snack food were recalled in the context of a 2007 outbreak associated with contamination from Salmonella serotype Wandsworth and Salmonella Typhimurium found in broccoli powder in the United States and Canada.[10]
Global Academic and Industrial Awareness
The aforementioned articles document governmental awareness and focus. However, greater awareness in the academic and industrial communities is growing, as evidenced by some recent articles on the topic (there are many more). These include the book The Microbiological Safety of Low Water Activity Foods and Spices,[11] the Grocery Manufacturers Association document Control of Salmonella in Low-Moisture Food[12] and the article “Sources and Risk Factors for Contamination, Survival, Persistence, and Heat Resistance of Salmonella in Low-Moisture Foods.”[13]
In a very large study performed by Sagoo et al.[14] in the UK, Salmonella spp. were detected in 1.5 percent of 132 production batches and 1.1 percent of 2,833 retail samples. Thus, the awareness of Salmonella in spices is worldwide. Furthermore, spices are frequently grown and shipped from outside the United States, often from third-world countries where spices are harvested and stored under conditions that don’t meet developed-world standards for hygiene and sanitation. For example, 75 percent of U.S. spice imports come from Brazil, China, India, Indonesia, Madagascar, Mexico, Peru and Vietnam.[11]
What the Industry Can and Should Do
This is the time for the spice industry and indeed the entire low water activity food industry, as well as other industries, to ensure that they have nothing less than a world-class food safety system. Such a system will include many things, such as but not limited to supplier controls, appropriate sanitization approaches—given that traditional wet-cleaning approaches have been shown to pose greater risk in dry food processing environments—preoperational swabbing programs for validation of the effectiveness of cleaning and sanitization, rigorous environmental sampling and corrective action programs, appropriate lot-based testing and segregation of processing lines, hygienic equipment design and repair practices, preventive maintenance programs and ongoing training.[15] Stay tuned for a forthcoming article discussing some of these approaches.
Jeffrey L. Kornacki, Ph.D., is president of Kornacki Microbiology Solutions Inc. in Madison, WI. He is on the Editorial Advisory Board of Food Safety Magazine.
References
1. Lynch, MF and RV Tauxe, “Salmonellosis: Non-Typhoidal,” in Bacterial Infections of Humans, eds. AS Evans and PS Brachman (New York: Springer, 2009).
2. Scallan, E et al. 2011. “Foodborne Illness Acquired in the United States—Major Pathogens.” Emerg Infect Dis 17(1):7–15.
3. www.cdc.gov/ncezid/dfwed/PDFs/epi101-508c.pdf.
4. Wiedmann, M. “Listeria Science and Issues.” Presentation at the 2016 Food Safety Summit. Rosemont, IL.
5. www.fda.gov/Food/FoodScienceResearch/WholeGenomeSequencingProgramWGS/ucm403550.htm.
6. Vij, V et al. 2006. “Recalls of Spices Due to Bacterial Contamination Monitored by the U.S. Food and Drug Administration: The Predominance of Salmonellae.” J Food Protect 69:233–237.
7. www.fda.gov/downloads/Food/FoodScienceResearch/RiskSafetyAssessment/UCM367337.pdf.
8. Ma, Y. 2013. “United States Food and Drug Administration: Review of Primary Recall Events During 2008–2009 Associated with Salmonella in Spices and Seasonings.” Personal communication. Available from FDA upon request.
9. www.cdc.gov/salmonella/2010/montevideo-5-4-2010.html.
10. Sotir, MJ et al. 2009. “Outbreak of Salmonella Wandsworth and Typhimurium Infections in Infants and Toddlers Traced to a Commercial Vegetable-Coated Snack Food.” Pediatr Infect Dis J 28(12):1041–1046.
11. JB Gurtler, MP Doyle and JL Kornacki. The Microbiological Safety of Low Water Activity Foods and Spices (New York: Springer, 2014).
12. www.gmaonline.org/downloads/technical-guidance-and-tools/SalmonellaControlGuidance.pdf.
13. Podolak, R et al. 2010. “Sources and Risk Factors for Contamination, Survival, Persistence, and Heat Resistance of Salmonella in Low-Moisture Foods.” J Food Prot 73(10):1919–1936.
14. Sagoo, SK et al. 2009. “Assessment of the Microbiological Safety of Dried Spices and Herbs from Production and Retail Premises in the United Kingdom.” Food Microbiol 26(1):39–43.
15. Kornacki, JL. “Hygiene Control in the Dry Food Products Industry: The Roles of Cleaning Methods and Hygienic Indicators,” in Case Studies in Food Safety and Authenticity: Lessons From Real-Life Situations, ed. J Hoorfar, 254–266 (Cambridge, UK: Woodhead Publishing, 2012).
The Coming Storm in the Spice Industry