For companies engaged in the manufacture or distribution of food products ensuring food safety is a key business activity. Most food companies have the basic elements of food safety, but fail to view these discrete elements as components of a comprehensive food safety system. Because of this narrow view of food safety, companies often experience difficulty in sorting through the financials and in providing appropriate funding for this critical aspect of the business. The management of these companies may have an inaccurate view of the effectiveness of their food safety efforts, as well, because the assessment of the food safety programs also is limited in scope rather than evaluated as a total system.
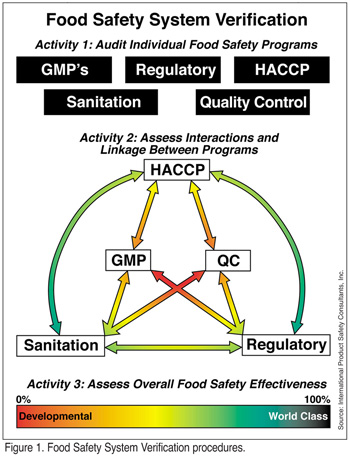 The ability of the food company to integrate its food safety verification procedures can provide a standard and uniform means for judging the overall effectiveness the food safety system. Company management should be provided with a precise, objective view of the company's food safety efforts in the form of a Food Safety System Verification (FSSV) process composed of three conjoined activities (Figure 1). Specifically, the FSSV process objectives are achieved by auditing the various elements of the overall food safety system. This "audit of the audits," if you will, provides a rating system that can be used to evaluate food safety efforts.
The ability of the food company to integrate its food safety verification procedures can provide a standard and uniform means for judging the overall effectiveness the food safety system. Company management should be provided with a precise, objective view of the company's food safety efforts in the form of a Food Safety System Verification (FSSV) process composed of three conjoined activities (Figure 1). Specifically, the FSSV process objectives are achieved by auditing the various elements of the overall food safety system. This "audit of the audits," if you will, provides a rating system that can be used to evaluate food safety efforts.
The three FSSV activities include an in-depth audit of each of the five fundamental elements (food safety programs) of the system; an evaluation of the component interactions and linkage; and an overall assessment of the food safety system's effectiveness. It is proposed that such a rating system will not only provide a professional, independent appraisal of the effectiveness of a company's overall food safety system and establish the degree of interaction and linkage amongst the allied elements of the food safety program, but also provide company management with an objective means for assessing and funding food safety and provide better information by which to direct continuous improvement in the area of food safety.
Step 1: Identifying the Program Areas to Be Audited
There are five key programs or elements that are fundamental to achieving food safety in the typical food processing operation. These rudimentary elements are the essential building blocks for developing a robust, reliable, sustainable and comprehensive food safety system. Each discrete program area is comprised of several activities or key performance indicators (KPIs) requiring that audits be conducted to ensure that each individual program is working properly. While it is true that these programs do not contain critical control points in the classical sense, it is also true that they each contain activities that are essential to achieving food safety. The first phase in the FSSV approach depends on identifying and assessing the key performance indicators subordinating each of the five programs in order to perform in-depth evaluations. In brief, these five programs are:
Hazard Analysis & Critical Control Points (HACCP) Procedures. This seven-principle, science-based approach for ensuring food safety has become an essential component of most food manufacturing operations, whether or not its implementation is mandated for a specific food category by regulation. The HACCP concept involves risk identification and the development of specific countermeasures to mitigate the hazard. The primary focus of the strategy is the control and elimination of hazardous biological, chemical and physical matter from the food supply.
Good Manufacturing Practices (GMPs). These procedures are designed to maintain industry, company and government standards of practice for manufacturing, holding and distributing foods fit for human consumption. GMP regulations are codified, for example, at Title 21, parts 110, 113 and 114 of the Federal Code of Regulations.
Quality Control (QC). This includes measures and procedures developed specifically by companies or corporations for ensuring cost-effective production of uniform and consistent products. Typically, QC programs are local-, product- and process-specific measures that are applied at the point of production. QC programs frequently consider the physical, chemical or organoleptic attributes of a food product. QC activities are focused on meeting product performance criteria established and agreed upon by product development and marketing.
Regulatory Compliance. This program ensures adherence to national legislation and other relevant international standards related to the production and distribution of foods fit for human consumption. Such regulations include the Federal Food Drug and Cosmetic Act; Federal Meat Inspection Act; Egg Inspection Act; The Federal Insecticide Fungicide and Rodenticide Act; or the standards of Codex Alimentarius.
Sanitation Procedures. This includes the programs and procedures related to cleaning and maintaining a hygienic work environment for producing human foods, such as the scheduling of cleaning activities to preclude the proliferation of undesirable microorganisms in the various unit operations of a manufacturing facility. Sanita-tion also includes procedures to identify the proper materials of construction for both food processing equipment and the food processing facility.
Often, the processor does not have the means of identifying and isolating weaknesses in the company's food safety program. Internal and external auditing are useful tools for troubleshooting and determining the effectiveness of a food safety system. Typically, food companies employ third-party auditing firms to provide a professional, objective evaluation of one or more of these five elements of the comprehensive food safety program.
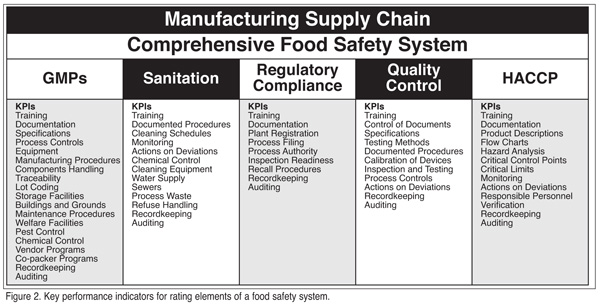 The independent auditing firms will provide criteria or key performance indicators for measuring attainment of the goals specific to each of the five program areas. Key performance indicators for each of the five food safety program areas, as identified by IPSC, are listed in Figure 2, and provide a good summary of the elements of each program that should be audited.
The independent auditing firms will provide criteria or key performance indicators for measuring attainment of the goals specific to each of the five program areas. Key performance indicators for each of the five food safety program areas, as identified by IPSC, are listed in Figure 2, and provide a good summary of the elements of each program that should be audited.
Each of the five programs that comprise the food safety system is rated individually by assessing the attainment of its subordinating KPIs. For example, the analysis of the KPIs specific to the GMP program is the measure of that program's effectiveness. Likewise for KPIs subordinating the Sanitation, Regulatory Comp-liance and HACCP programs. The KPI analysis requires an in-depth audit of each of the subordinating activities for each of the five program areas. If one were performing this analysis on a company's GMP program, the auditor might conclude, for example, that the traceability efforts were deficient because of the failure to consider and track the utilization rework. Similarly, the auditor might report that the traceability program was excellent with regard to tracking finished product, but lacking in its ability to track specific lots of the ingredients or other components used in the manufacture of the company's finished product.
In another example, the auditor's report on the evaluation of the sanitation program may indicate the existence of inadequate procedures to confirm the complete removal of sanitation chemicals from a system that has undergone a clean-in-place (CIP) cleaning procedure. The same auditor also might conclude that the sanitation chemicals were not compatible with the product or residue type, and therefore note that the system is not being properly cleaned or sanitized. The same audit report might also indicate that, based on available records, sanitation workers are not properly trained in the handling of sanitation chemicals. Based on this analysis, a total KPI score can be provided for each component of the program, reflecting the relative effectiveness of the specific element. The assigned KPI rating will identify a breach in procedures or a general loss of overall program integrity.
It is critical that the auditing firm work with food company management and plant personnel to coordinate, plan and execute the audits. It is highly recommended that the processor obtain an audit plan from the auditing firm in advance of the audit. This plan provides the protocol and procedures used by the auditors, identifies specific records and other documents subject to request during the course of the audit, and establish and identify the auditing standards. A pre-audit meeting with key plant and corporate personnel should be arranged for the day of the actual audit. The meeting will highlight the audit schedule and procedures. Similarly, at the conclusion of the audit, an exit interview is convened to review the preliminary findings and to identify data or other materials that are required for completion of the evaluation process.
During the audit, at least one company representative should accompany the auditor. The company representative(s) should be knowledgeable of plant operations and have the authority to answer questions related to manufacturing practices, processes and related procedures.
At minimum, the auditor can be expected to review the following: procedures for calibration and testing of measuring devices; inspection and testing practices; training programs; storage and warehouse procedures; ingredients receiving and handling practices; refuse disposal practices; process controls procedures; processing equipment design and hygiene; HACCP plans; documentation; record keeping; corrective actions for process or procedural deviations; sanitation program and procedures; in-plant chemical management protocol; pest management procedures; buildings and facilities; water supply; and process waste handling; welfare facilities and locker rooms; and plumbing and sewer plans. The auditor will also inquire after licenses, and proof of registration and compliance with applicable state and federally mandated programs related to food production and distribution.
Once the KPI ratings have been established for each program, a more in-depth evaluation can commence.
Step 2: Evaluating Program Interaction and Linkage
The second activity in a food safety system verification process involves an evaluation of the food safety component interactions and linkage. This activity provides a comprehensive analysis of the management process. Generally, the linkage evaluation is the most difficult to objectively self-evaluate due to the political nature of any organization. The auditor should determine how effectively each component interacts, or even overlaps, through an assessment of customer and supplier relationships, management involvement, joint protocols and procedures, and joint execution of corrective actions.
In addition to the four categories listed above, the linkage rating also is based on the auditors findings related to reciprocal sign-off procedures, mutual involvement with development and maintenance of specification and other documents, team development, and involvement and contribution to the continuous improvement process. This information will be gleaned from records, reports, general observations and discussions with representatives from the various departments involved with the comprehensive food safety program.
The fundamental assumption underpinning the linkage rating is that the interactions between the elements of the program can either be negative, non-existent or positive. For example, in an environment characterized by the existence of functional silos, it would be reasonable to expect either negative (antagonistic) or nonexistent relationships between the various programs when different supervisors or managers head them up. By contrast, in an open, linear working environment one would likely find that the programs were very complementary with many shared elements of responsibility.
While the five individual elements of the food safety system are rated as a function of their interaction with each other, all program elements are not expected to interact with each of the others to the same degree. For example, the interactions of the QC and the GMP programs will differ as compared to the QC and HACCP components with respect to achieving food safety. The fact is that the focus of the activities (KPIs) amongst the various programs will vary with respect to their contribution to the food safety program. That is, the KPIs for the GMP program have a significant focus on preventing product contamination and thereby in promoting food safety. By contrast, the typical QC program usually has little or no focus on preserving the public health aspects food processing operations. Thus, the relationship between GMP program and QC program, while significant, will not be as highly rated as the relationship between the GMP and HACCP program for achieving food safety objectives. This difference is reflected in the linkage rating. A high degree of integration corresponds with maturity, timely execution and efficacy of the entire food safety program. Too high of an integration rating can indicate areas of duplicate effort and excess. Mastery of the component interaction process indicates a cost-effective food safety program with minimum risk.
In all, a total of 10 pairs of elements are considered and scored. Certain interactions between the elements of the program are more important than others in terms of predicting the overall effectiveness of the food safety system. Therefore, each of the interacting couplets is weighted to reflect their relative importance to the program. For example, it is obvious that the interactions between the GMP program and the HACCP program are far more important to achieving food safety than are the linkages between the QC and Regulatory programs. Hence, in rating these two couplets, the former would be assigned a higher overall rating. Thereby, reflecting the value of its contribution to the food safety system.
Step 3: Overall Assessment
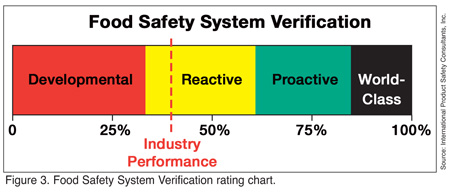 The results from these program components and linkage interaction assessments now can be combined into the Food Safety System Verification rating (Figure 3). This value reflects the overall performance and effectiveness of the food safety system. The rating is based both on KPI performance and the degree of linkage among the elements in the food safety system. The KPI performance is more important overall than is the analysis of program interactions. The KPI analysis is an expression of overall planning and development of the food safety system. Linkage analysis is an estimation of the effective of management in its administration of the system. Robust systems often will compensate for poor management. This fact is reflected in the FSSV rating, which identifies the relative effectiveness of the overall system's ability for containment of food safety risk.
The results from these program components and linkage interaction assessments now can be combined into the Food Safety System Verification rating (Figure 3). This value reflects the overall performance and effectiveness of the food safety system. The rating is based both on KPI performance and the degree of linkage among the elements in the food safety system. The KPI performance is more important overall than is the analysis of program interactions. The KPI analysis is an expression of overall planning and development of the food safety system. Linkage analysis is an estimation of the effective of management in its administration of the system. Robust systems often will compensate for poor management. This fact is reflected in the FSSV rating, which identifies the relative effectiveness of the overall system's ability for containment of food safety risk.
The FSSV rating is divided into four broad categories:
• Developmental: Systems not fully developed, and are therefore unreliable for identification or containment of food safety risk. High degree of associated risk.
• Reactive: Good general program development, but not fully executable for risk management. Moderate associated risk.
• Proactive: Excellent program development, which is fully executable for both risk identification and management. Low associated risk.
• World-Class: Best practices and excellent programs overall. Minimum associated risk.
By way of example, consider an auditor's findings that show a highly developed HACCP program that is linked to weak or underdeveloped constituent programs. Under this scenario, the food processor would be assigned an overall FSSV rating of Developmental, indicating that the company is at risk of a significant food safety failure due to both poorly developed programs and poor management. High scores for both the KPI analysis and linkage analysis would characterize a Proactive rated food safety system, which is both anticipatory and prepared to respond in preventing a food safety failure.
The verification step exposes vulnerabilities and demonstrates strengths in the overall program. Areas of concern are identified and easily prioritized for improvement. Evaluating risk exposure and allocating resources can be greatly facilitated by using this standardized and uniform method for rating the effectiveness of a company's food safety efforts.
Advantages of Auditing Your Audits
Just as food safety infers far more than HACCP, auditing should be viewed as far more than just the verification of CCPs. HACCP is a single food safety strategy in a broader, more comprehensive program directed at ensuring the safety of a company's products. Integrating the information derived from auditing each single element of the five manufacturing support programs commonly employed in today's food manufacturing operations can provide a better view of the effectiveness of the company's total food safety system.
In addition to appraising the elements of the food safety system, the FSSV approach to auditing also provides a view to the effectiveness of the management in administering the elements of the system. This approach to auditing provides management a multidimensional view to the effectiveness of the company's food safety efforts. Such a strategy provides greater protection for the company's brands, reputation, consumers and earnings.
In fact, the desire of food processors to develop strategies that identify and manage food safety risk throughout the entirety of the manufacturing supply chain has never been greater, as evidenced by recent increases of dedicated food safety professionals within more food companies. Food companies have always endeavored to make and market safe products, but food safety, as a discipline within the industry is a reasonably new development.
Traditionally, it has been assumed within the industry that quality assurance also does food safety. However, it must be understood that food safety and quality are very different propositions. Quality is negotiable; food safety is not. Moreover, a catastrophic food safety failure will portend devastating economic consequences and cause irreparable harm to the business. Quality failures seldom result in the acute, overt failure of a business.
The integrated Food Safety System Verification approach to auditing the essential elements of the food safety system increases the confidence of those charged with growing the business and meeting the expectation of the corporation's shareholders. Confidence that will enable innovation and increase the speed to market with new products and technologies. Confidence that will permit expansion of a company's supply chain into new markets and potentially result in a lowering of manufacturing costs. Confidence that consumers will have confidence in your brands. Confidence that enables growth of the business and increased earnings.
Read the sidebars "On-Site Audits Key to Verifyting Quality Systems for Bakery’s", "Seafood Processor Issues Audit Challenge to Industry" and "NFPA Supplier Audit Program Reaches 100th Audit Milestone"
Larry Keener is the founder and general manager of the Seattle, WA-based consulting frim, International Product Safety Consultants (IPSC). Founded in 1996, IPSC specializes in food safety, microbiology, regulatory affairs and crisis management. Keener has nearly 30 years of experience in the food processing industry, holding positions in private industry, with the State of California and at the National Food Processors Association. He has worked extensively in the development and implementation of food safety programs around the world. Prior to founding IPSC, Keener served as the director of Product Safety and Regulatory Affairs for one of Unilever's largest food companies worldwide. He has a long history of involvement, at the national level, in dealing with a wide range of food industry issues.
Keener is the executive director of the Non-Thermal Processing Alliance, and IPSC is the U.S. representative for CSIRO's Food Science Australia. He is an active member of the Institute of Food Technologists and the International Association of Food Protection, and serves on the Editorial Advisory Board of the internationally distributed scientific trade publication, Food Safety Magazine. He can be reached via e-mail at lkeener@aol.com or on the web at www.foodsafetyprofessionals.com.
On-Site Audits Key to Verifyting Quality Systems for Bakery’s
Following rigorous qualifications and audits, Chicago, IL-based East Balt Inc., a dedicated McDonald's Corp. supplier of hamburger buns and English muffins, achieved HACCP accrediation at their Chicago bakery from Guelph Food Technology Center and the American Institute of Baking (www.aibonline.org).
"This process has instilled a sense of accomplishment and pride in the continuous improvement of our quality system for manufacturing buns and muffins for McDonald's Corp.," said John Petennes, president of East Balt. "We touch 10 million McDonald's customers each week with our products and we want only the highest standards of food safety and quality maintained every day."
Prior to the HACCP accreditation process, AIB International provided verification of GMP compliance, HACCP prerequisite programs, manual plan review, training and implementation by an on-site audit of all systems. The successful completion of a two-day HACCP verification audit proved icing on the cake in terms of what East Balt learned during the HACCP accreditation process.
"The focus on HACCP criteria and employee training has taught us so much about how to develop and maintain systems with a focus on each component to make us better at our jobs," noted Louis Kurchuris, HACCP Team Coordinator. "We all learned so much about our quality system that we took for granted, and that we had to think through every system in great detail. It makes you step back and take a new look at what you are really doing every day, and how much detail is involved in food safety and quality systems. It has definitely made us better as employees and as a company."
With the HACCP accreditation, East Balt has completed one of six qualification steps toward fulfilling the rigorous criteria required for the AIB Bakers Quality Seal.
Seafood Processor Issues Audit Challenge to Industry
A recent report issued by the U.S. Food and Drug Administration (FDA) generated concern from a consumer watchdog group about the quality of food safety standards used in the seafood industry. In sharp contrast to how the FDA findings are being presented to the public, Ducktrap River Fish Farm recently released the results of an independent food safety audit that verifies high safety standards.
NSF-Cook & Thurber (www.nsf.org) conducted the comprehensive audit of Ducktrap River Fish Farm's smoked seafood processing plant in late June and gave the Belfast, ME facility a food safety rating of 93.5%. This is criteria of "Beyond Practical Improvement."
"We challenge any one of our smoked seafood competitors to match our ratings performance with an inspection as intense as the one performed by NSF-Cook & Thurber," said Steve Young, vice president of sales and marketing for Ducktrap River Fish Farm., which takes seafood from environmentally sustainable farmed and wild fisheries and creates more than 35 unique smoked seafood products. The products contain no nitrates, nitrites or artificial preservatives.
The purpose of the audit was to evaluate the presence of food safety and quality systems and verify by independent inspection that the systems were operating as designed and under control. In addition to the food safety rating, the company also received a 92.73% plant audit score.
The auditor, Barry Tancred, stated in his report that the seafood processing facility is "a very modern and well run plant that has been constructed with food safety in mind....plant construction and design facilitate the production of product meeting customer and regulatory food safety and quality requirements."
"The standards for cold smoked, ready-to-eat seafood are much higher than for other cooked proteins. That's why we invest considerable time and money into designing and implementing systems that ensure our products are the safest and highest quality in the industry," said Young.
NFPA Supplier Audit Program Reaches 100th Audit Milestone
The National Food Processors Association (NFPA) is pleased to announce that Supplier Audits for Food Excellence, known as the NFPA-SAFE Program, has contracts for 100 supplier audits at the midway point in its first year of operation.
"The NFPA-SAFE Program has taken hold in the food industry and is poised to become the standard for supplier audits in North America," said Rich Salotto, Vice President of the NFPA-SAFE Program. "Confidence in our positioning relates both to rapid achievement of the 100th audit milestone, as well as commitments by major food processing companies to this process," Salotto said.
The NFPA-SAFE Program is designed to allow suppliers to satisfy multiple customers with a standardized audit carried out by SAFE-certified, third-party auditors. The SAFE audit is a comprehensive assessment of a company's entire quality and food safety system, covering five main categories: management responsibility, fundamentals, food safety systems, quality systems, and regulatory compliance.
Several major manufacturers, including General Mills, Heinz and Kraft Foods, are systematically notifying their suppliers that the NFPA-SAFE Program will be required in order to secure and maintain vendor agreements.
"General Mills recently aligned itself with other major food companies in adopting the NFPA-SAFE auditing service to replace our periodic supplier inspections," the company wrote to suppliers. "This comprehensive audit will help standardize food safety requirements and provide benefits to both you and to General Mills. The benefits to you include a reduced number of redundant inspections by General Mills and many other food processors, standardized inspection criteria, and a more thorough quality focused audit," wrote Dave Sorenson, Vice President, Worldwide Sourcing and Jon Blake, Vice President, Quality and Regulatory.
Also, Heinz North America is requiring its most critical suppliers to conduct an NFPA-SAFE Program audit in 2002. In a letter from the head of Heinz procurement and Catherine Adams, Ph.D., R.D., General Manager, Quality Assurance, Heinz wrote to its top-priority vendors: "The audit is rigorous and comprehensive, as you would expect Heinz and other customers to require. There are a number of national and multinational companies who may accept the NFPA audit in fulfillment of their supplier audit requirements. We encourage you to determine if your other customers will accept the NFPA audit."
Kraft Foods is requiring its suppliers to schedule an audit by the NFPA-SAFE Program in order to continue to achieve its high quality standards. According to Jean Spence, Senior Vice President of Worldwide Quality, Scientific Affairs & Compliance, "Kraft has full confidence in the NFPA-SAFE Program, and we expect our ingredient suppliers to participate."




