As a basic requirement of both Hazard Analysis and Critical Control Points (HACCP) and Preventive Controls for Human Food (PCHF) regulations, the performance and documentation of a thorough hazard analysis is the cornerstone of food safety plans. There are various definitions of hazard analysis from Codex, the U.S. Department of Agriculture (USDA), and the U.S. Food and Drug Administration (FDA),[1–5] essentially stating the same thing. The Food Safety Preventive Controls Alliance PCHF training manual defines a hazard analysis specifically as “the process of collecting and evaluating information on hazards and conditions leading to their presence to decide which are significant for food safety and therefore must be addressed in the HACCP or food safety plan.”[6] When conducting a hazard analysis, potential biological, chemical, or physical food safety hazards that are reasonably foreseeable to cause illness or injury are identified so that controls or preventive measures can be determined and implemented through the food safety plan.[7] It is important to note that these hazards may come from ingredients or develop through conditions associated with the manufacturing process. Therefore, depending on the length of the ingredient list and the complexity of the manufacturing process, it could be beneficial to conduct the ingredient hazard analysis and the process hazard analysis separately.
Considerations in the Hazard Analysis
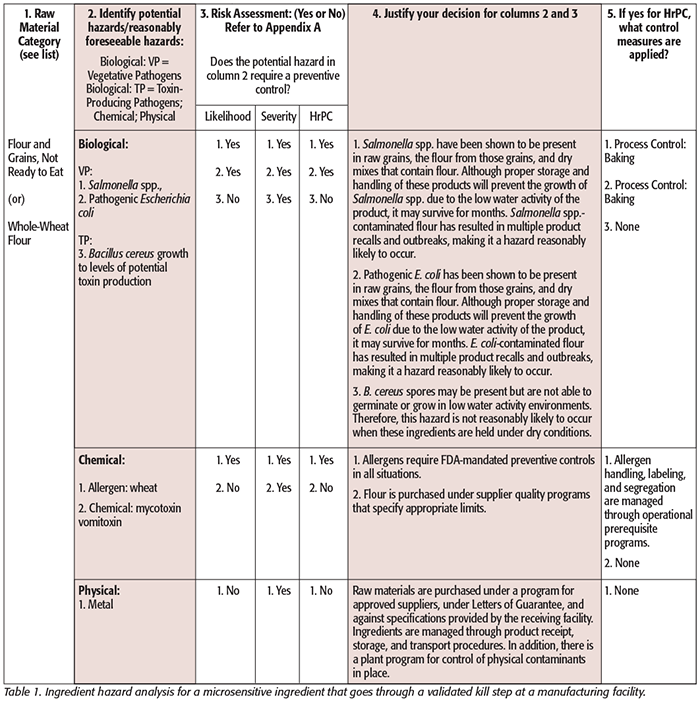
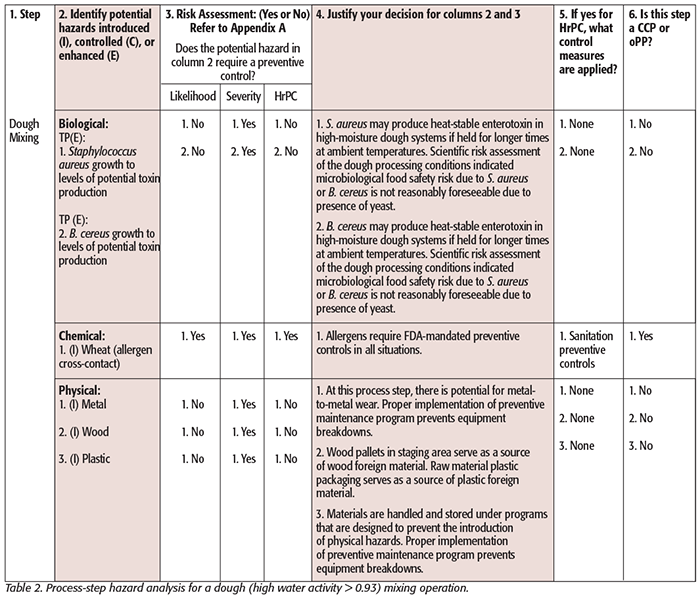
While hazard analysis formats vary slightly from one agency to another, two basic elements are contained in both: hazard identification and hazard evaluation. Tables 1–3 illustrate generic examples of hazard analysis work sheets applicable to different jurisdictions (Tables 1 and 2: PCHF; Table 3: FDA – Juice HACCP[8]). Tables for FDA – Seafood HACCP and USDA can be found here.[9] These examples are provided for illustration purposes and are based on published guidance. You can see that the following components must be considered and included in each: a listing of each ingredient or ingredient category/process step, the potential hazards associated with each ingredient or process step, a risk analysis of each ingredient or process step that includes an assessment of the severity and likelihood of occurrence of the hazard, and a justification or rationale for each of the decisions made. This process leads the food safety team to conclude whether the analyzed hazards require a preventive control.
Before conducting the hazard analysis, ensure that the process flow diagram is complete and no steps are overlooked. A written description is useful to explain what happens at each of the process steps and can contain more detail than the flow diagram. Be as specific as you can in your description. It is important to know what occurs at each process step and any underlying environmental conditions. For example, include information such as the maximum length of time that a high-moisture slurry/product could be exposed to unrefrigerated temperatures, a brief description of rework activities (especially if multiple allergens are handled), or identification of the handling and storage practices because these may impact food safety. Thus, a detailed description with specific process parameters and/or observations is important for an accurate hazard identification.
During the hazard identification for ingredients or a process step, leveraging scientific resources is necessary to justify the decisions made and will facilitate a conversation with an auditor or inspector about why certain hazards were or were not included in the food safety plan. These resources can include: Appendix 1 of the Hazard Analysis and Risk-Based Preventive Controls for Human Food: Draft Guidance for Industry, data involving recalls and outbreaks, peer-reviewed technical documents, regulatory guidance documents, and expert opinion. When determining whether a hazard may pose a significant risk to the consumer, a consideration of the severity of the illness or injury and the likelihood of occurrence is made. Evaluation of severity is influenced by consideration of several factors, such as the magnitude and duration of illness or injury, any chronic sequelae, and susceptibility of different subpopulations (e.g., children versus young healthy adults versus elderly). The likelihood of occurrence of the hazard takes into consideration several factors that include the formulation of the food, raw materials and ingredients, storage and distribution of the food, the intended or reasonably foreseeable use, equipment sanitary design, processing procedures, and any other relevant factors.

One common approach for the hazard identification and analysis is to have a single form that lists the ingredients within the context of receiving-process steps and then proceed to list the remaining process steps that include whether the hazard is introduced, controlled, or enhanced. Another approach is to have two separate forms, one for the hazard identification/analysis for ingredients, and the other for hazard identification/analysis for process steps. This second approach will be discussed in more detail.
Ingredient Hazard Analysis
When the hazard analysis has been separated into two distinct sections, ingredient hazard analysis and process hazard analysis, the determination of whether a reasonably foreseeable hazard is introduced, controlled, or enhanced does not necessarily apply in each hazard analysis section. For the ingredient hazard analysis, the team needs to consider whether this hazard is inherent to this material and whether it is reasonably foreseeable that this material may carry this hazard. Whether a hazard is controlled or enhanced would not apply in the ingredient hazard analysis—this comes into play in the process hazard analysis along with introduced. Looking at it this way helps conduct the hazard analysis in a more logical, thorough, and stepwise manner.
Process-Step Hazard Analysis
In the process-step hazard analysis, the team must consider what hazards could be introduced, controlled, or enhanced at each process step. Some might consider a hazard introduced during receiving as one that is getting introduced into a facility on an ingredient. As will be discussed later, this may be acceptable but may not be preferred because it can obscure the more insightful/relevant thought process of the process-step hazard analysis. Looking at it slightly differently, consider whether the activity(ies) at the specific process step does the introducing, the controlling, or the enhancing. Therefore, the act of receiving will not introduce a hazard unless you accidentally or routinely breach package integrity often upon receipt of ingredients (through sample collection, for example) or use bulk off-loading. Another example of a reasonably foreseeable introduced hazard at a process step is the possibility of metal shavings during grinding. An example of an enhanced hazard is when there is work in process where high moisture, time, and temperature are factors allowing pathogenic microorganisms that may be present to multiply.[10] Finally, the team would consider a process step as controlling a hazard if it significantly minimizes or prevents the hazard.
Hazard Analysis Templates: Pros and Cons
With both ingredients and process steps in a single hazard analysis form, there may be an advantage of only having to create and maintain a single hazard analysis document in the HACCP/food safety plan. The document will most certainly be shorter in length! However, the main disadvantage of this approach is that it is often very easy to disregard, or even miss, assessing certain hazards associated with the ingredient-receiving steps, because the hazard analysis tends to focus on the hazards inherent to the ingredient being received and not the process step of receiving that ingredient. For example, using this approach, the step of receiving raw milk would list the biological (pathogen) and chemical (allergen, drug residues) hazards inherent in raw milk. But the process of off-loading the milk could introduce physical hazards that are not necessarily inherent in the milk. Another example would be receiving bulk salt by air-blowing the salt from a tanker into a silo. This step would typically not list any biological or chemical hazards, because these are not usually associated with the salt, but the process of blowing the salt could introduce biological hazards if unfiltered air is used. If it is a small operation, there could be additional hazards with receiving ingredients by manually transferring them from one container into another, where it is possible to miss the potential biological hazard (bacterial pathogens, viruses) from employees’ handling of the ingredient.
If separate forms are used for the hazard analysis of ingredients and process steps, initially there will be more work needed to create the documentation because there will now be two separate hazard analyses conducted. Nevertheless, the main advantage of doing a hazard analysis on ingredients separate from process steps is the completeness of hazard identification. When identifying hazards inherent in ingredients, all potential hazards may be considered for each ingredient, and a thorough hazard analysis is performed for each one. The same can be said for the process-step hazard analysis. Only hazards that are created by the performance of each process step are analyzed, without any confounding factors carrying over from the ingredient analysis. Using the examples above, raw milk as an ingredient would be assessed for its inherent hazards only in the ingredient hazard analysis (biological and chemical), and the raw milk-receiving step would list hazards created only by the act of receiving that milk, such as potential physical hazards created by off-loading. The same can be said for the bulk salt ingredient. In the ingredient hazard analysis, there would most likely be no hazards associated with the salt, but there could be potential biological and physical hazards listed in the salt-receiving step because of the way the salt was being removed from the truck.
In the event of major changes (e.g., new ingredients, allergens, addition/deletion of process steps due to decommissioning of products) to the food safety plan, separate forms of hazard analysis (ingredients versus process steps) offer the food safety team the opportunity to perform comprehensive hazard analysis and identification of appropriate preventive controls. Consider a scenario where a manufacturer of peanut butter-filled pretzels decided to also produce chocolate-covered peanut butter-filled pretzels. The facility’s food safety team can clearly identify microbial hazards and chemical hazards inherent to chocolate on the ingredient hazard analysis. Also, an allergen line assessment can be performed using the process flow to identify reasonably foreseeable hazards at different process steps. This will enable the food safety team to perform a reanalysis of the HACCP/food safety plan and identify appropriate preventive controls/measures. Furthermore, the food safety team can easily communicate the effectiveness of the facility’s programs in the event of an audit or regulatory inspection. Using the same example above, a combined hazard analysis form may obscure the hazard identification process at the process steps and may solely focus on ingredients.
Conclusion
Science-based identification and evaluation of hazards will lead to the identification of preventive controls that will significantly minimize or prevent hazards that can cause illness or injury to the consumer. Addressing hazards that are inherent to ingredients separately from hazards that may be introduced, controlled, or enhanced during processing may help the food safety team more thoroughly analyze potential hazards and determine the most effective controls for prevention (or reduction). An ineffective hazard analysis may lead the food safety team to focus on hazards that are not reasonably foreseeable, resulting in a waste of resources. More importantly, an insufficient hazard analysis can lead to inadequate identification or implementation of preventive controls, which could eventually cause a domino effect leading to the failure of the food safety system as whole. Therefore, an accurate and complete hazard analysis is vital to the development of a comprehensive HACCP or food safety plan designed to protect public health.
References
1. Codex Alimentarius Commission. HACCP System and Guidelines for Its Application – Annex to CAC/RCP 1-1969, Rev. 3 (1997).
2. USDA Food Safety and Inspection Service, 9 C.F.R. Part 417, HACCP Final Rule. July 1996.
3. FDA Seafood: Fish and Fishery Products Hazards and Controls Guidance, 4th ed. August 2019.
4. FDA Guidance for Industry: Juice HACCP Hazards and Controls Guidance, 1st ed. March 2004.
5. FDA Hazard Analysis and Risk-based Preventive Controls for Human Food: Draft Guidance for Industry. August 2016.
6. FSPCA – Preventive Controls for Human Food Training Curriculum. February 2016.
7. FDA. 2015. Federal Register. “Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food,” Final Rule; Response 93; Pages 55949–55950.
8. foodscience.psu.edu/facilities/creamery/food-safety-procedures-and-documents/creamery-haccp-and-safety-plans/Juice-HACCP-Plan.pdf.
9. www.food-safety.com/magazine-archive1/augustseptember-2020/ingredients-hazard-analysis-and-process-step-hazard-analysis-why-separate-the-two/.
10. www.food-safety.com/magazine-archive1/december-2018january-2019/time-flies-when-temperatures-rise/.
Balasubrahmanyam Kottapalli Ph.D., Director, Enterprise Food Safety and Microbiology, at Conagra Brands.
Nancy Dobmeier CQA, CHA, A principal microbiologist, Enterprise Food Safety and Microbiology, at Conagra Brands.
Loralyn H. Ledenbach M.Sc., Principal scientist at The Kraft Heinz Company.